Terlipressin acetate preparation and preparation method thereof
A technology of terlipressin and acetic acid, used in lighting and heating equipment, pharmaceutical formulations, drying solid materials without heating, etc., can solve problems such as poor stability, achieve low impurity content, good stability, meet transportation and Effects of storage requests
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
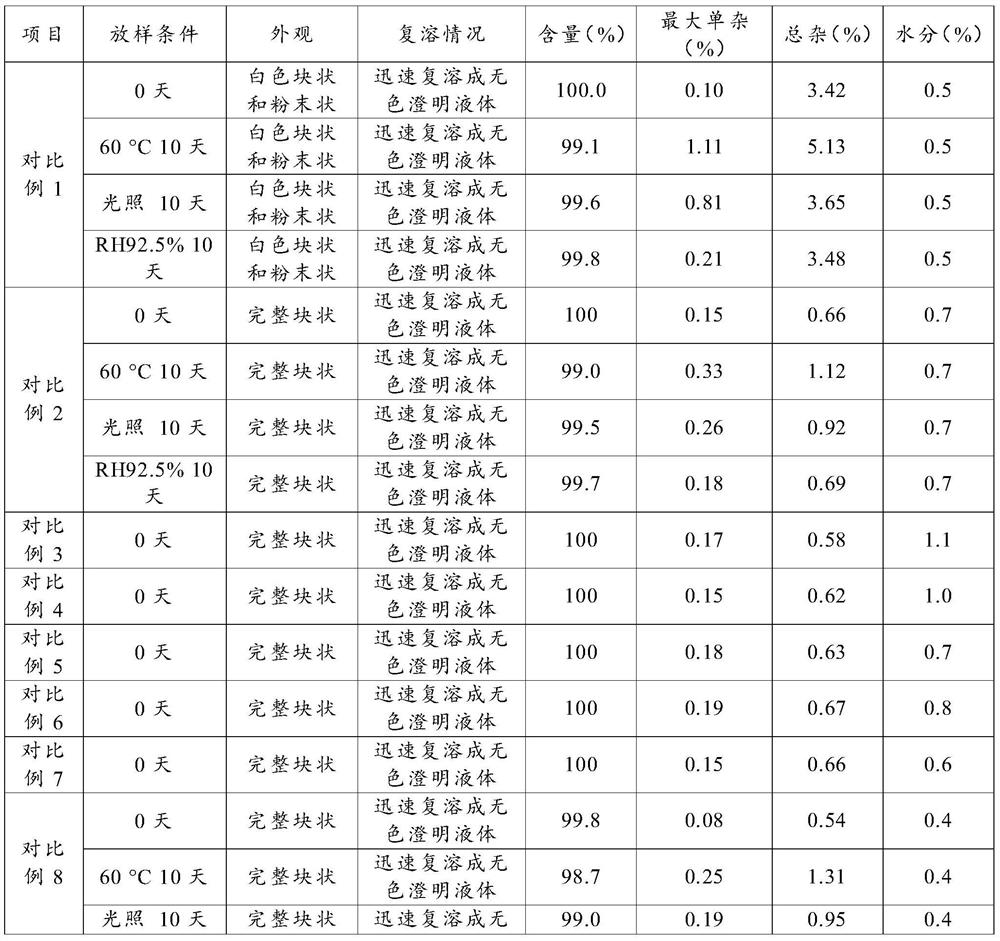
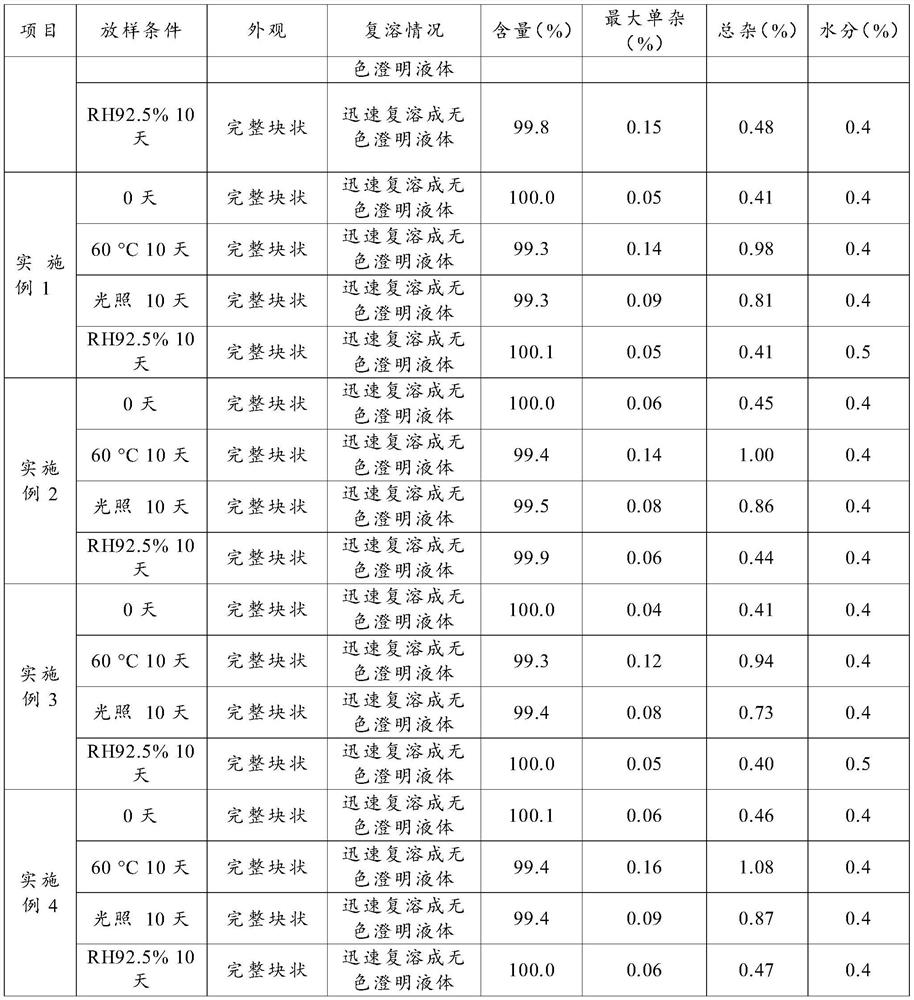
Examples
Embodiment 1
[0044] A preparation method of terlipressin acetate preparation, comprising the following steps:
[0045] (a) Preparation of mixed solution: Measure 40L of water for injection in the liquid preparation tank, weigh 470g of mannitol and add it to the water for injection, stir to make it completely dissolve; weigh 43g of terlipressin acetate raw material until adding the mannitol solution , stir to dissolve it; weigh 105g of dextran and add it to the liquid medicine, stir to dissolve it; use 1mol / L hydrochloric acid solution to adjust the pH to 3.8, and use water for injection to set the volume to 50L;
[0046] (b) Intermediate detection: take an appropriate amount of intermediate medicinal liquid, and measure the content of intermediates and other indicators;
[0047] (c) Filtration: use a 0.22 μm polyvinylidene fluoride filter membrane to filter and sterilize;
[0048] (d) Filling: according to the test results of the intermediate liquid medicine, fill the liquid medicine into...
Embodiment 2
[0063] A preparation method of terlipressin acetate preparation, comprising the following steps:
[0064] (a) Preparation of mixed solution: Measure 40L of water for injection in the liquid preparation tank, weigh 431g of mannitol and add it to the water for injection, stir to make it completely dissolve; weigh 42g of terlipressin acetate raw material until adding the mannitol solution , stir to dissolve it; weigh 107g of dextran and add it to the liquid medicine, stir to dissolve it; use 1mol / L hydrochloric acid solution to adjust the pH to 4.0, and use water for injection to set the volume to 50L;
[0065] (b) Intermediate detection: take an appropriate amount of intermediate medicinal liquid, and measure the content of intermediates and other indicators;
[0066] (c) Filtration: use a 0.22 μm polyvinylidene fluoride filter membrane to filter and sterilize;
[0067] (d) Filling: according to the test results of the intermediate liquid medicine, fill the liquid medicine into...
Embodiment 3
[0082] A preparation method of terlipressin acetate preparation, comprising the following steps:
[0083] (a) Preparation of mixed solution: Measure 40L of water for injection in a liquid preparation tank, weigh 950g of mannitol and add it to the water for injection, stir to make it completely dissolve; weigh 88g of terlipressin acetate raw material until adding the mannitol solution , stir to dissolve it; weigh 223g of dextran and add it to the liquid medicine, stir to dissolve it; use 1mol / L hydrochloric acid solution to adjust the pH to 3.0, and dilute the volume to 100L with water for injection;
[0084] (b) Intermediate detection: take an appropriate amount of intermediate medicinal liquid, and measure the content of intermediates and other indicators;
[0085] (c) Filtration: use a 0.22 μm polyvinyl chloride filter membrane to filter and sterilize;
[0086] (d) Filling: according to the test results of the intermediate liquid medicine, fill the liquid medicine into a 7m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com