Controlled-volume infusion device
a technology of infusion device and controlled volume, which is applied in the direction of flow monitor, intravenous device, medical syringe, etc., can solve the problems of inability to reuse for subsequent dosage, inconvenient use in alternate care sites such as the patient's home, and high cost of electronic pumps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
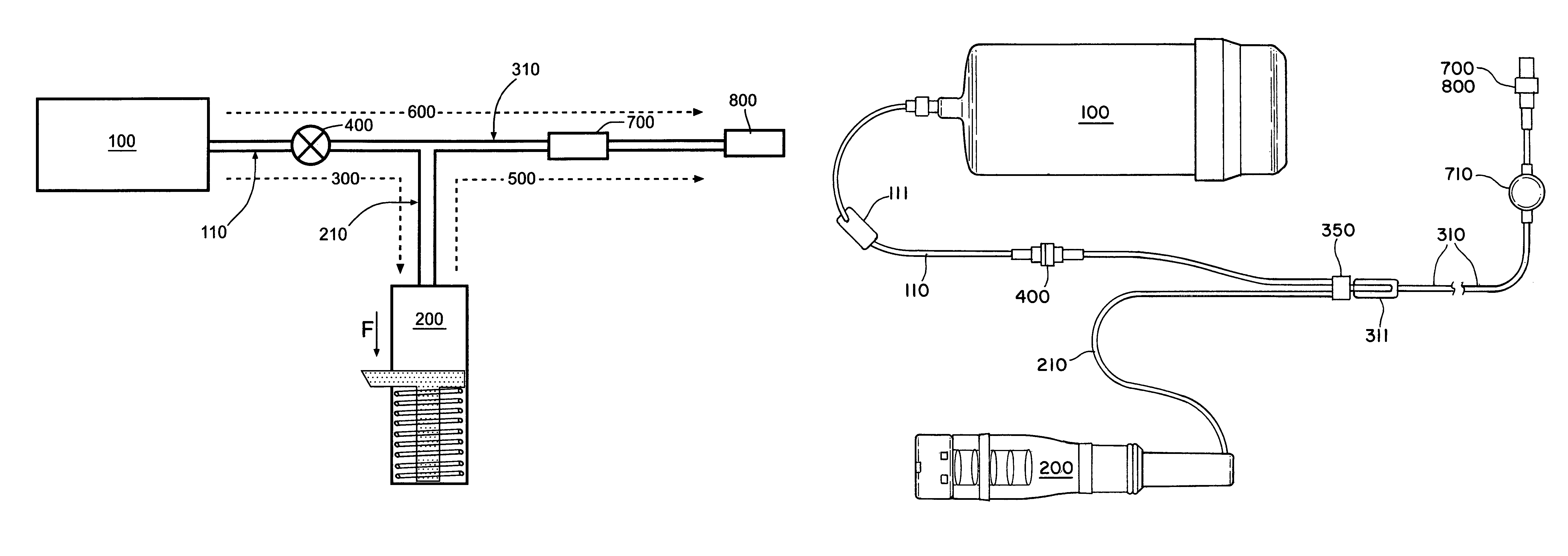
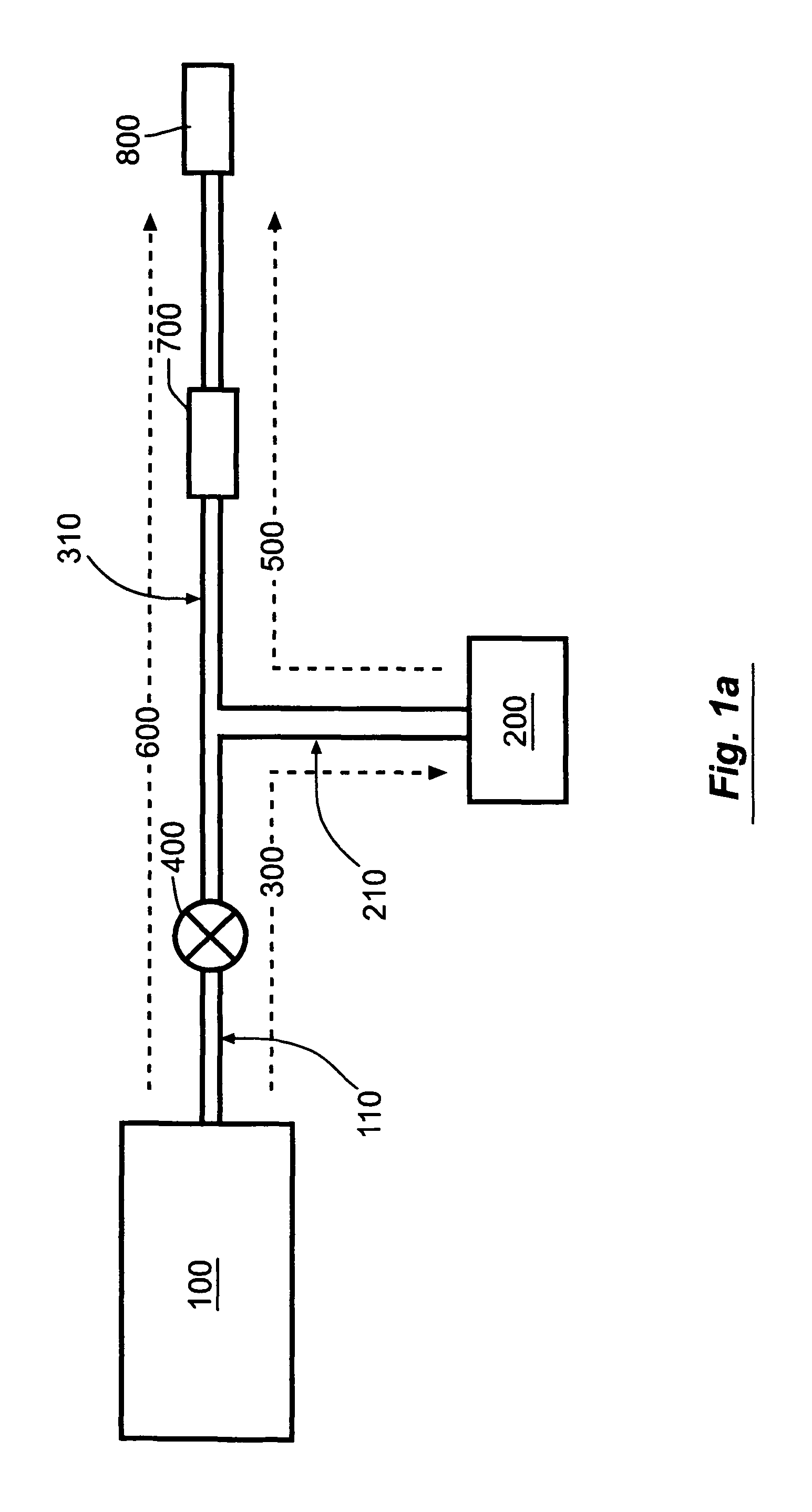
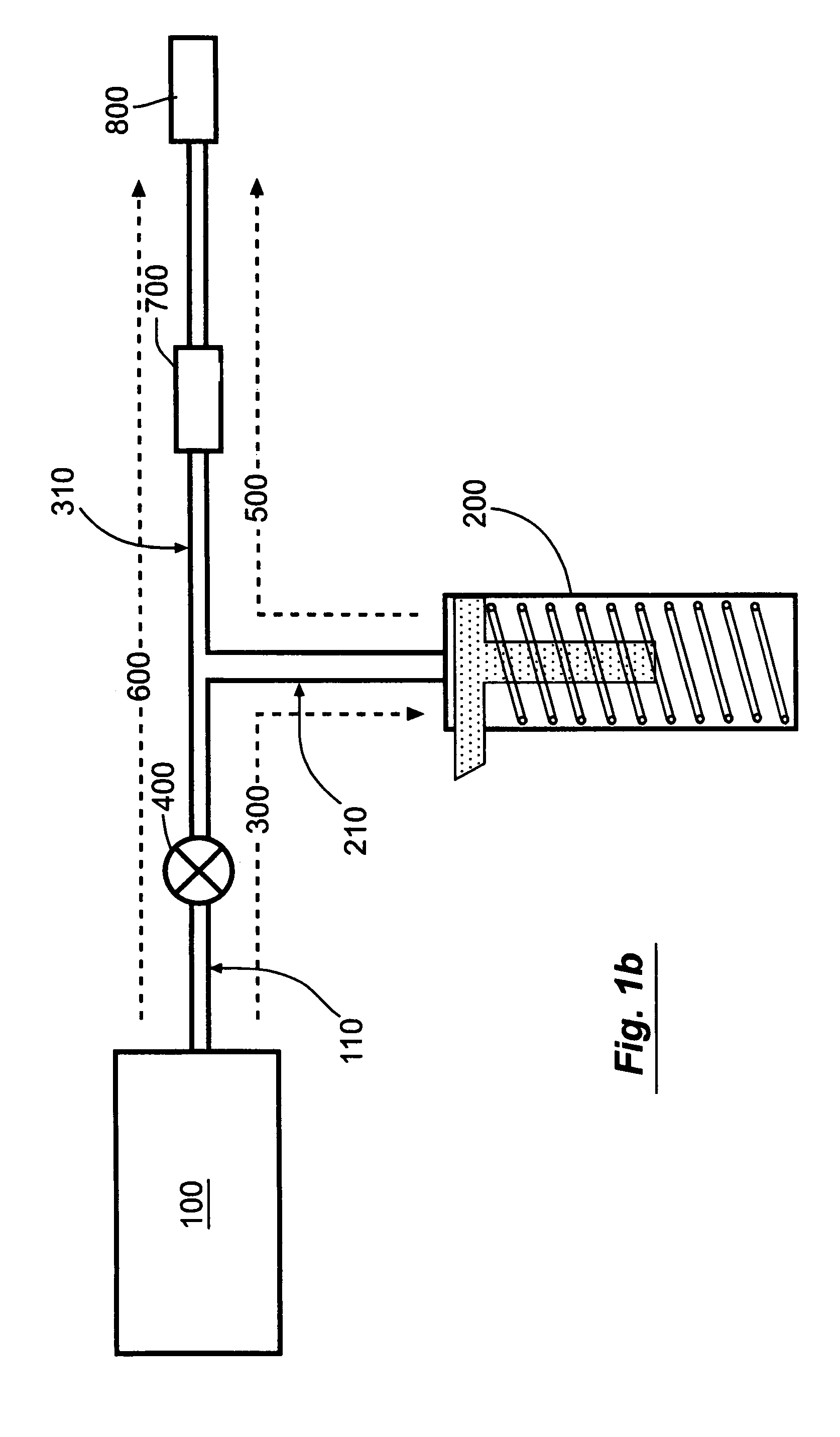
[0058]FIG. 1a shows a schematic view of the “basal-bolus” embodiment of the invention, providing for administration of a series of controlled-volume dosages of medication or other fluid with a continuous flow of fluid between dosages. The device includes a medication reservoir 100 that holds medication or other fluid under substantially constant pressure (the medication reservoir pressure, Pm). When the user actuates the controlled-volume dosage, the medication reservoir pressure causes fluid to be expelled from the medication reservoir 100, and to flow through the first fluid flow path 300 through the source conduit 110 and dosage conduit 210 into the controlled-volume dosage reservoir 200. When the dosage reservoir 200 is filled (as illustrated in FIG. 1c), the fluid within the dosage reservoir 200 is pressurized to a higher substantially constant pressure (the dosage reservoir pressure, Pd, which is not necessarily constant), which is greater than the medication reservoir pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com