Dosing regimens for treatment of proliferative disorders comprising administration of sapacitabine
a technology of proliferative disorders and dosing regimens, which is applied in the direction of biocide, drug compositions, animal husbandry, etc., can solve the problems of single-strand dna breakage that cannot be repaired, and achieve excellent anti-tumour activity, improved storage stability and ease of handling, and desirable pharmacokinetic profile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
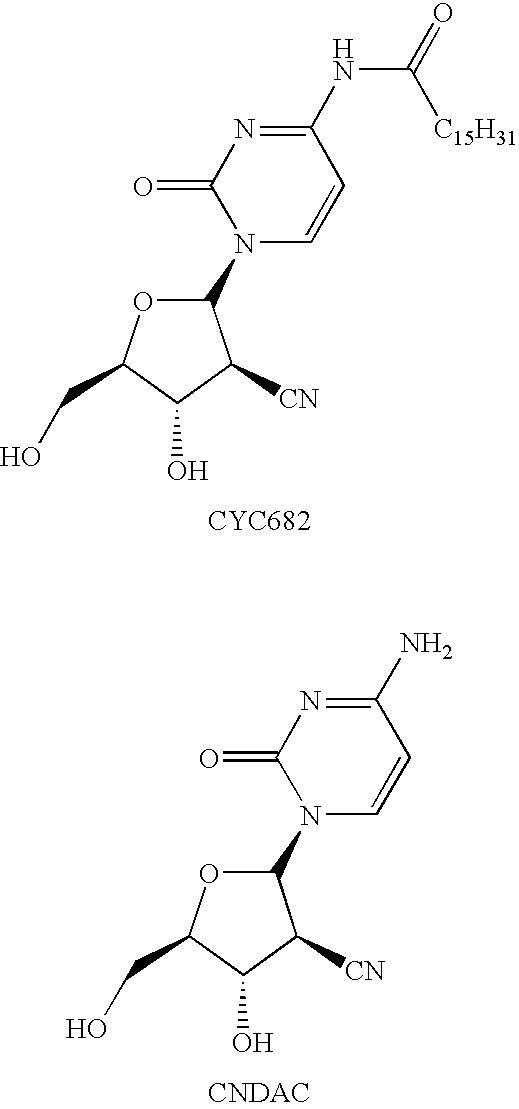
[0234]The B-form of sapacitabine was prepared in accordance with the methodology described in EP 536936 and EP 1364959, both in the name of Sankyo Company Limited.
Capsule Preparation
[0235]Liquid fill capsules were prepared in accordance with the methodology described in PCT / GB2006 / 004927 (WO 2007 / 072061; Cyclacel Limited).
[0236]The drug is supplied as 25 mg and 75 mg opaque white, gelatin capsules. This formulation comprises liquid-filled capsules of a sapacitabine-B crystalline form in miglyol 812N. Capsules are packaged in high-density polyethylene bottles (50 capsules per bottle), with low-density polyethylene screw-cap, child-resistant closures. The higher strength was formulated to fill into a size 1 capsule, while the lower strength was formulated to fill into a size 3 capsule as appropriate. All materials are of pharmacopoeial quality. A summary of the formulation components is provided in the table below.
[0237]
Formulation Capsule (mg / capsule)Unit FormulaIngredient25 mg75 mgS...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com