Cathode active material, cathode and nonaqueous secondary battery
a secondary battery and active material technology, applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of high price, low degree of existence of cobalt in earth's crust, thermal runaway reaction in the battery, etc., and achieve safety and cost. the effect of excelling and excelling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
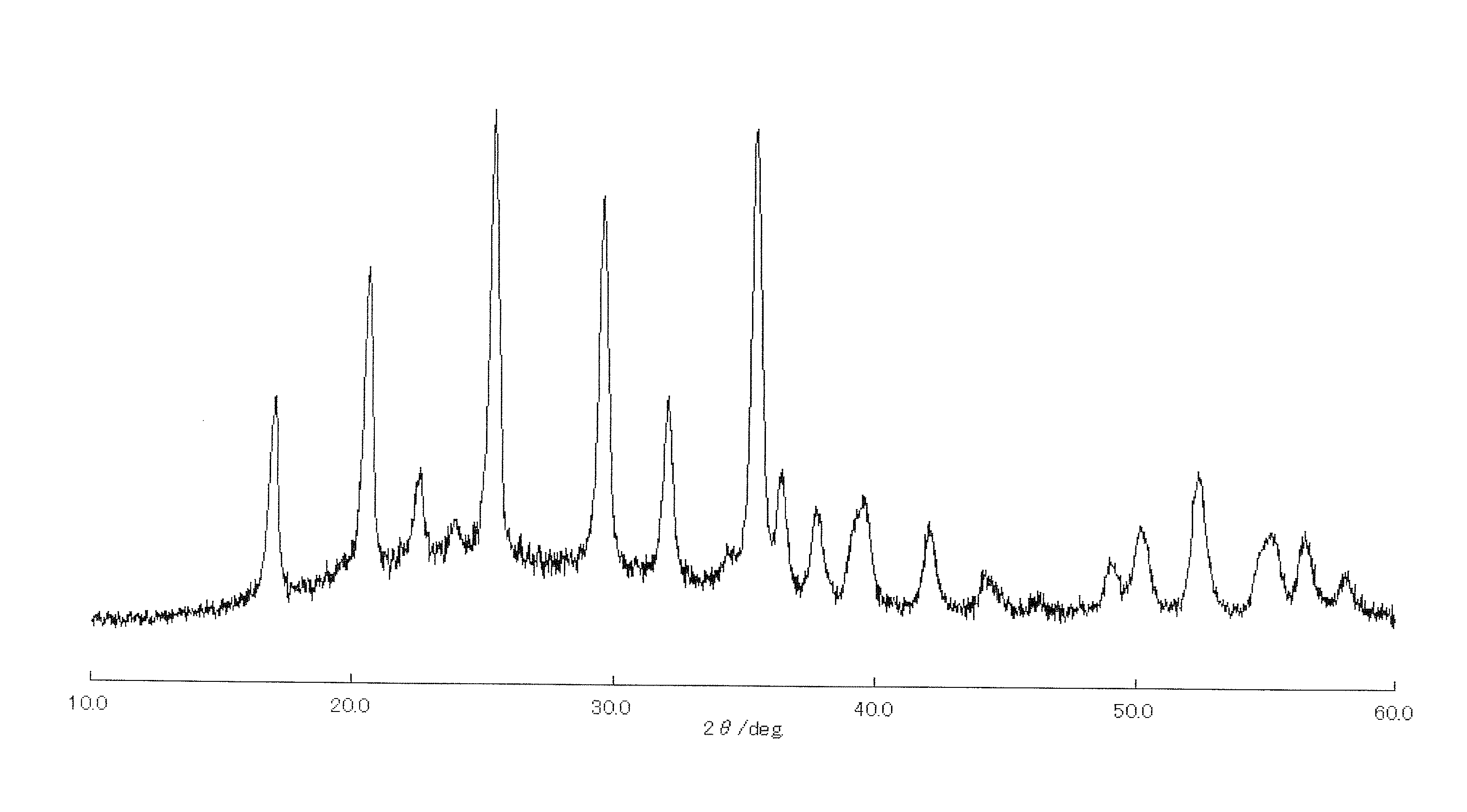
[0169]A lithium source Li(OC2H5), an iron source Fe(CH3COO)2, a zirconium source Zr(OC2H5)4, a phosphate source (NH4)2HPO4, and a silicon source Si(OC2H5)4 were used as starting materials, and these starting materials were measured out so that the molar ratio was Li:Fe:Zr:P:Si=1:0.875:0.125:0.75:0.25. Next, the Li source, the Zr source, and the Si source were dissolved in 20 g of butanol. Further, the Fe source and the P source were dissolved in water whose number of moles was four times as large as that total number of moles of metal alcoxide (the Fe source, the Si source, and the Li source). After one hour of stirring of a mixture of the butanol, in which the metal alcoxide had been dissolved, and the water, in which the Fe source and the P source had been dissolved, the resulting mixture was dried at 60° C. in a dryer into a powdery precursor.
[0170]The resultant amorphous precursor was sintered for twelve hours at 600° C. in a nitrogen atmosphere. Thus synthesized was LiFe0.875Zr...
example 2
Preparation of a Cathode Active Material
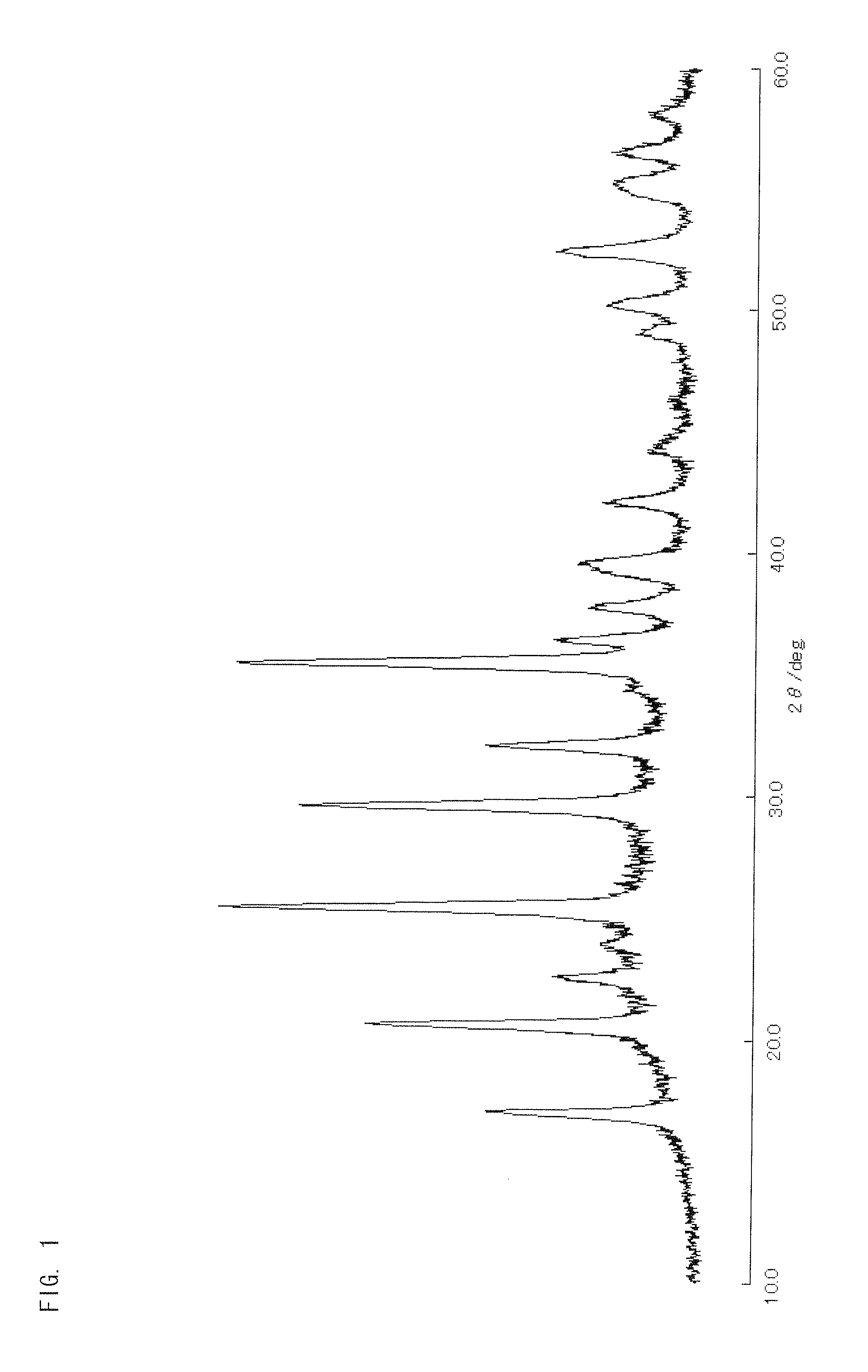
[0171]A lithium source LiCH3COO, an iron source Fe(NO3)3.9H2O, a zirconium source ZrCl4, a phosphate source H3PO4 (85%), and a silicon source Si(OC2H5)4 were used as starting materials. These starting materials were measured out so that the molar ratio is Li:Fe:Zr:P:Si=1:0.75:0.25:0.5:0.5, with the lithium source LiCH3COO used in an amount of 1.3196 g. These starting materials were dissolved in 30 ml of C2H5OH and stirred by a stirrer for 48 hours at room temperature. After that, the solvent was removed at 40° C. in a constant-temperature bath, with the result that a brownish-red powder was obtained.
[0172]After addition of 15 percent by weight of sucrose relative to the resultant powder, they were mixed well in an agate mortar, and the resulting mixture was pressure-molded into pellets. The pellets were sintered for twelve hours at 600° C. in a nitrogen atmosphere. Thus synthesized was LiFe0.75Zr0.25P0.5Si0.5O4 single-phase powder. The resulta...
example 3
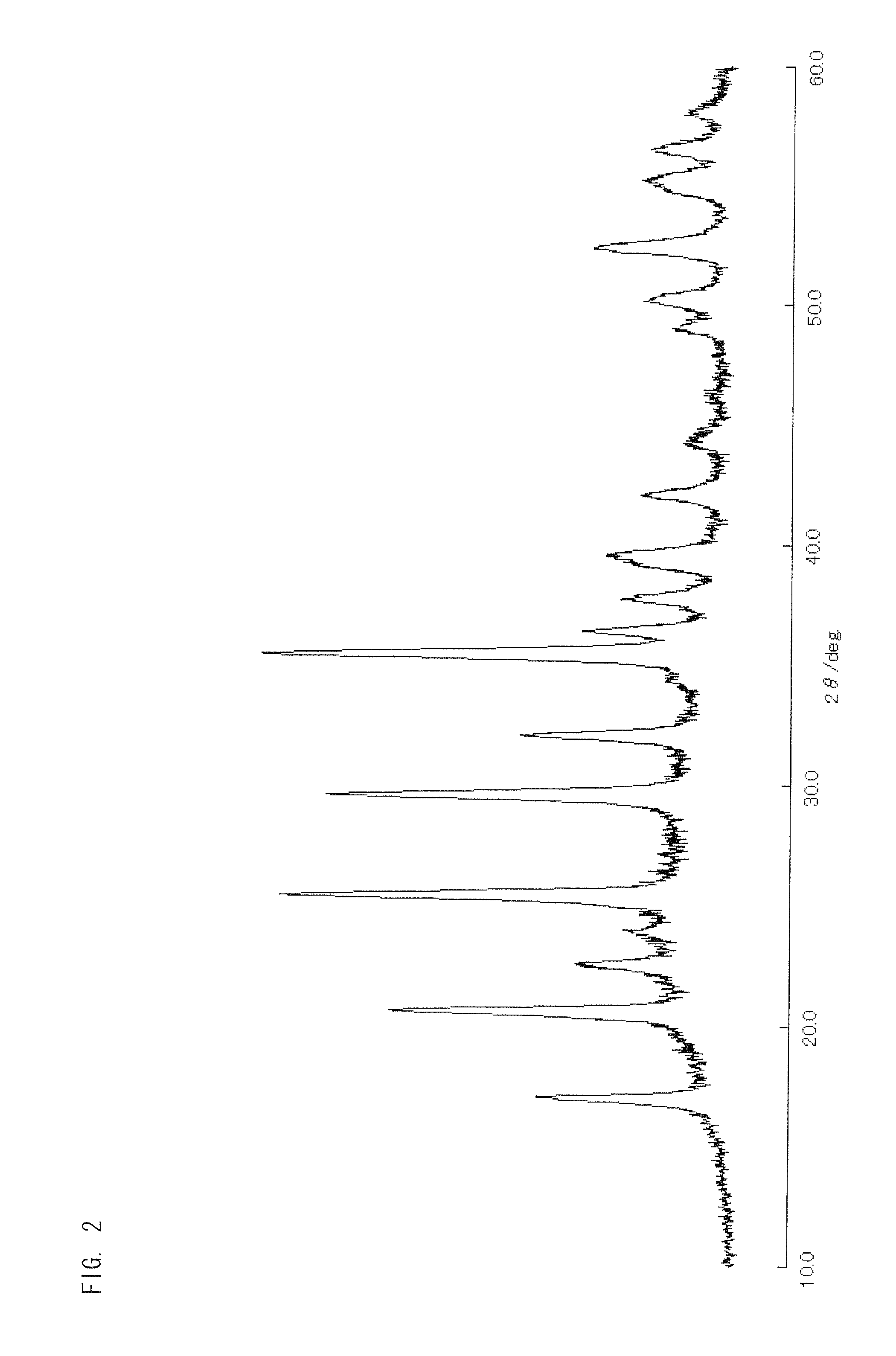
[0176]A lithium source LiCH3COO, an iron source Fe(NO3)3.9H2O, a zirconium source ZrCl4, a phosphate source H3PO4 (85%), and a silicon source Si(OC2H5)4 were used as starting materials. These starting materials were measured out so that the molar ratio is Li:Fe:Zr:P:Si=1:0.85:0.15:0.7:0.3, with the lithium source LiCH3COO used in an amount of 1.3196 g. These starting materials were dissolved in 30 ml of C2H50H and stirred by a stirrer for 48 hours at room temperature. After that, the solvent was removed at 40° C. in a constant-temperature bath, with the result that a brownish-red powder was obtained.
[0177]After addition of 15 percent by weight of sucrose relative to the resultant powder, they were mixed well in an agate mortar, and the resulting mixture was pressure-molded into pellets. The pellets were sintered for twelve hours at 600° C. in a nitrogen atmosphere. Thus synthesized was LiFe0.85 Zr0.15P0.7Si0.3O4 single-phase powder. The resultant cathode active material is referred ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com