Inhibiting the development of tolerance to and/or dependence on an addictive substance
a technology of addictive substances and tolerance, applied in the field of addictive substances, can solve the problems of toxicity of mk-801, unsuitable pharmaceutical use, limited long-term administration, etc., and achieve the effect of alleviating withdrawal symptoms and reducing withdrawal symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
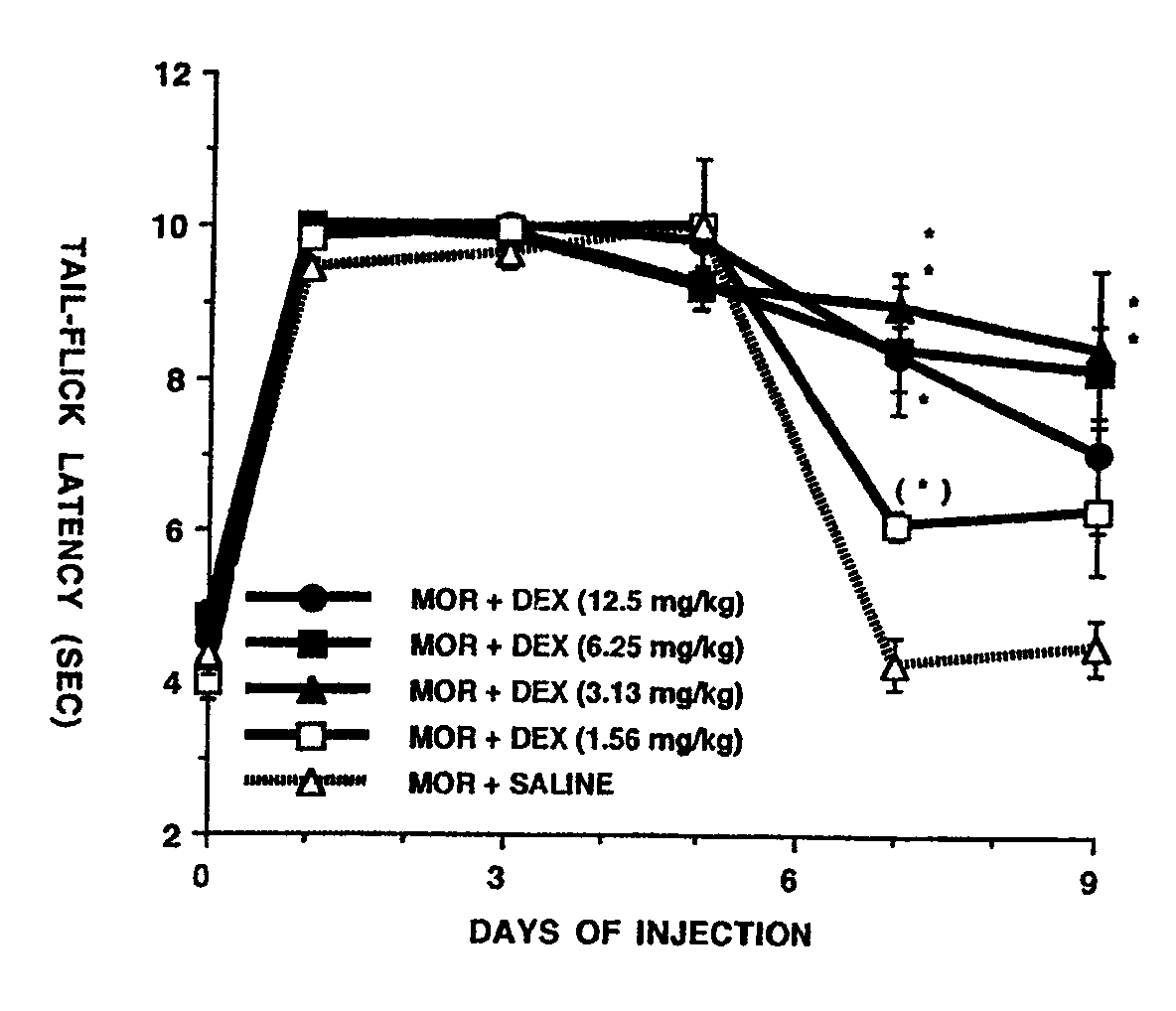
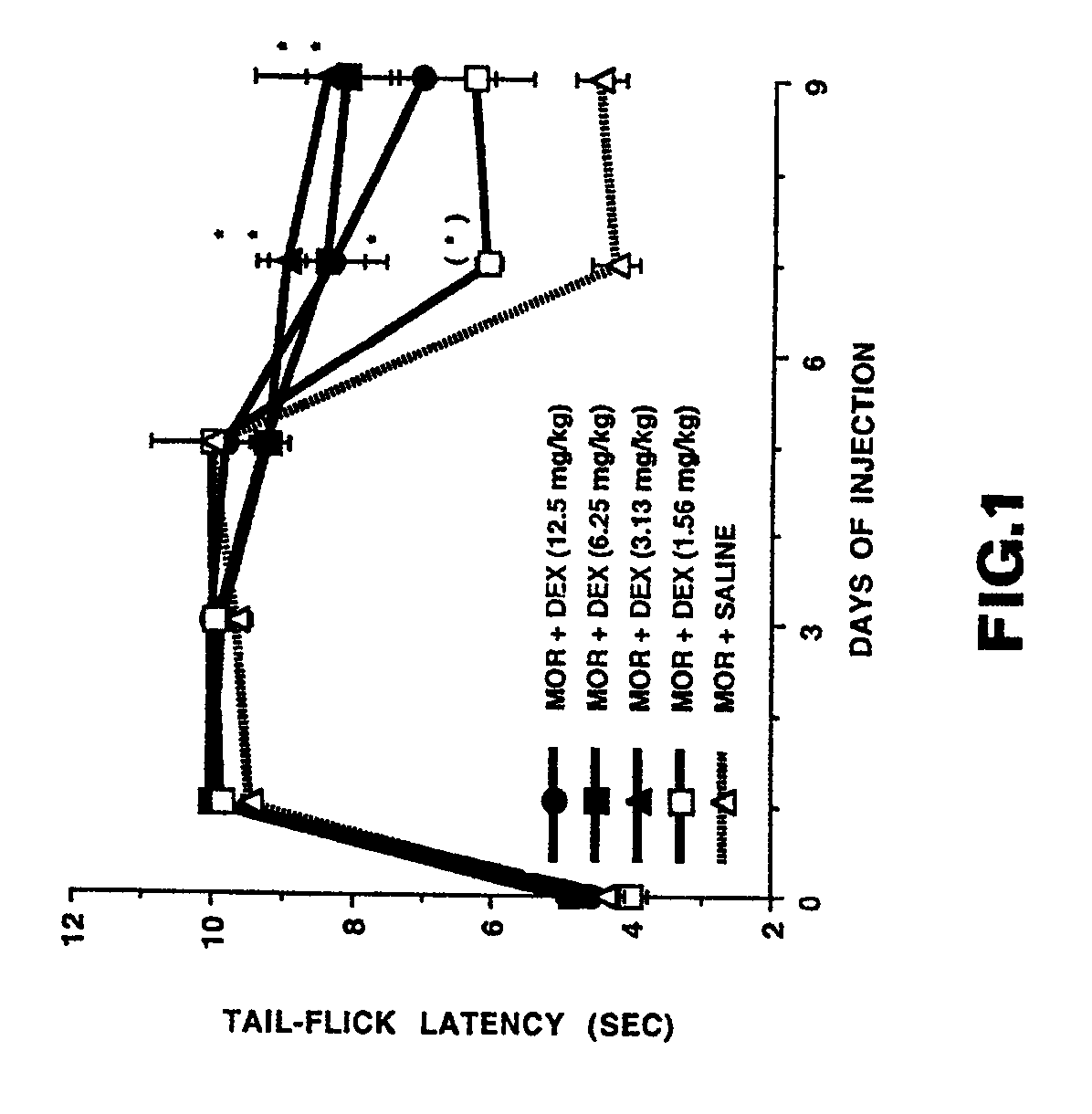
[0031]The effect of systemic dextrorphan on prevention of the development of morphine tolerance and dependence was examined in Sprague-Dawley rats weighing 350-400 gm. Morphine tolerance was developed in the rats by twice daily subcutaneous injection of 10 mg / kg morphine sulfate. The analgesic effect of the morphine was examined by using the well known tail-flick test which measures the latency of tail-flick upon radiant heat stimulation. The latency of tail-flick test is defined as the time elapsed from the onset of radiant heat to the flick of the rat's tail. In order to examine the effect of dextrorphan on the development of morphine tolerance, each morphine-treated rat also received intraperitoneal administration of either dextrorphan (1.56, 3.13, 6.25, 12.5 mg / kg, n=5 / group) or saline (n=6) given 30 minutes prior to each morphine administration.
[0032]FIG. 1 shows the effects of systemic doses of dextrorphan (DEX) on tolerance to morphine analgesia produced by twice daily subcut...
example 2
[0034]The effects of ganglioside GM1 in inhibiting morphine tolerance and dependence utilizing both systemic and intrathecal treatment were evaluated. The systemic treatment procedure, including both morphine and ganglioside GM1 administration, was exactly the same as that used in the experimental work presented in Example 1 except that ganglioside GM1 was given 1 hour before each morphine administration.
[0035]As shown in FIG. 3, the tail flick latency in ganglioside GM1-treated (10, 30, 60 mg / kg, n=6 / group) rats remained significantly longer than that of saline-treated rats on days 5, 7, 9, and 10 of repeated morphine administration, indicating the prevention of the development of morphine tolerance by ganglioside GM1. Although all 3 doses of ganglioside GM1 were effective, 30 and 60 mg / kg were more effective at days 9 and 10 than 10 mg / kg.
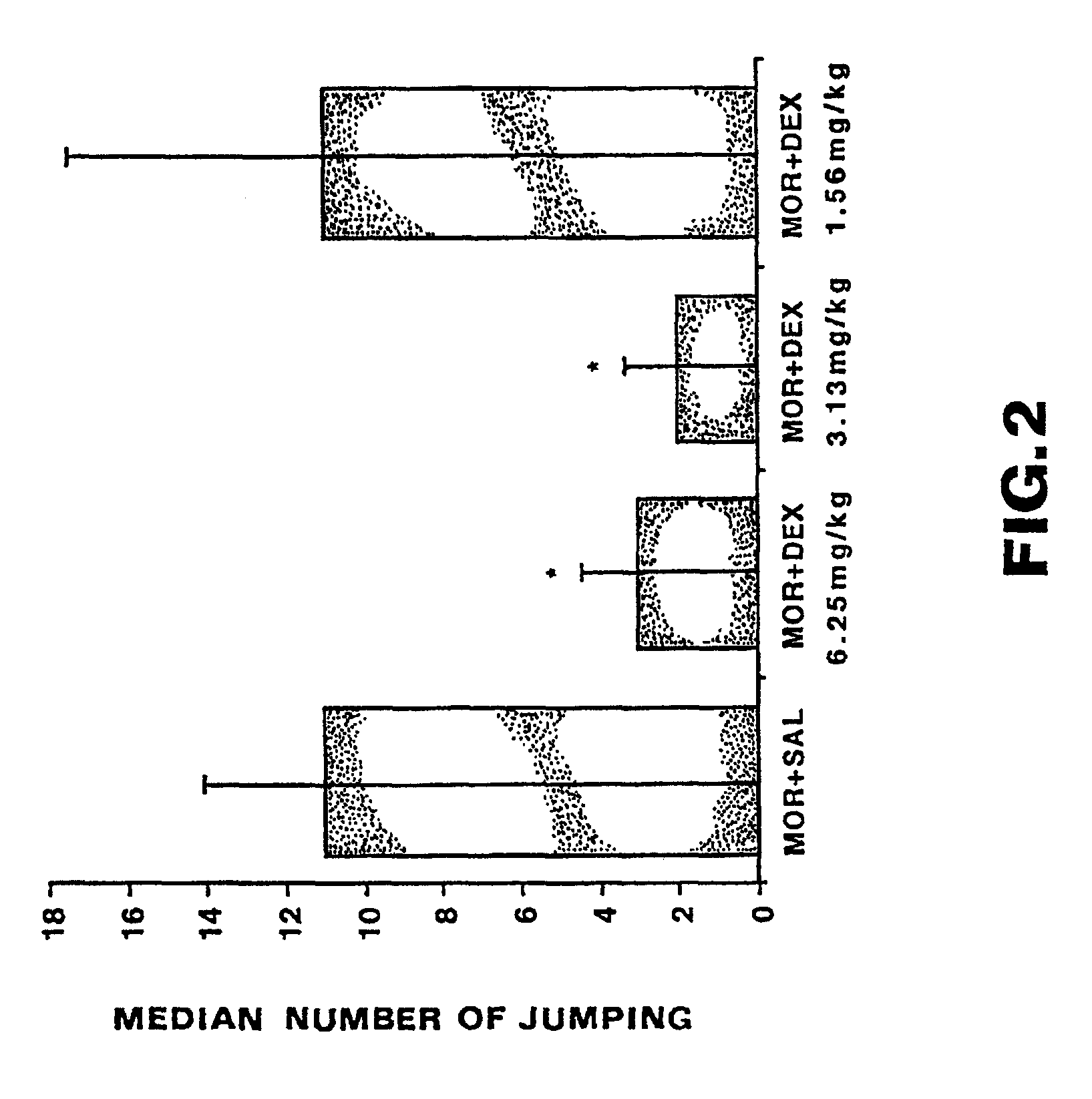
[0036]FIG. 4 shows the effects of systemic doses of ganglioside GM1 on jumping, a withdrawal symptom produced by subcutaneous naloxone (2 mg / kg)...
example 3
[0037]This example demonstrates the effectiveness of ganglioside GM1 in preventing the development of morphine tolerance at the site of the spinal cord. Morphine sulfate 10 μg was delivered once daily through an intrathecal (spinal) canula implanted 5 days before the first morphine injection. Ganglioside GM1 or saline also was delivered intrathecally 30 minutes before each morphine injection.
[0038]FIG. 5 shows the effects of the intrathecal doses of ganglioside GM1 on tolerance to morphine analgesia produced by once daily intrathecal administration of 10 μg morphine. Intrathecal ganglioside GM1 was given 30 min before each morphine administration. Each symbol represents mean scores for maximal possible effects (and hence analgesia) for each group of rats (n=5-6) measured at 15, 30, 60, 90, 120, 180, and 240 minutes after morphine injection on Day 8, i.e., 24 hours following 6 consecutive daily intrathecal morphine injections. Vertical bars are standard errors. Maximal possible effec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com