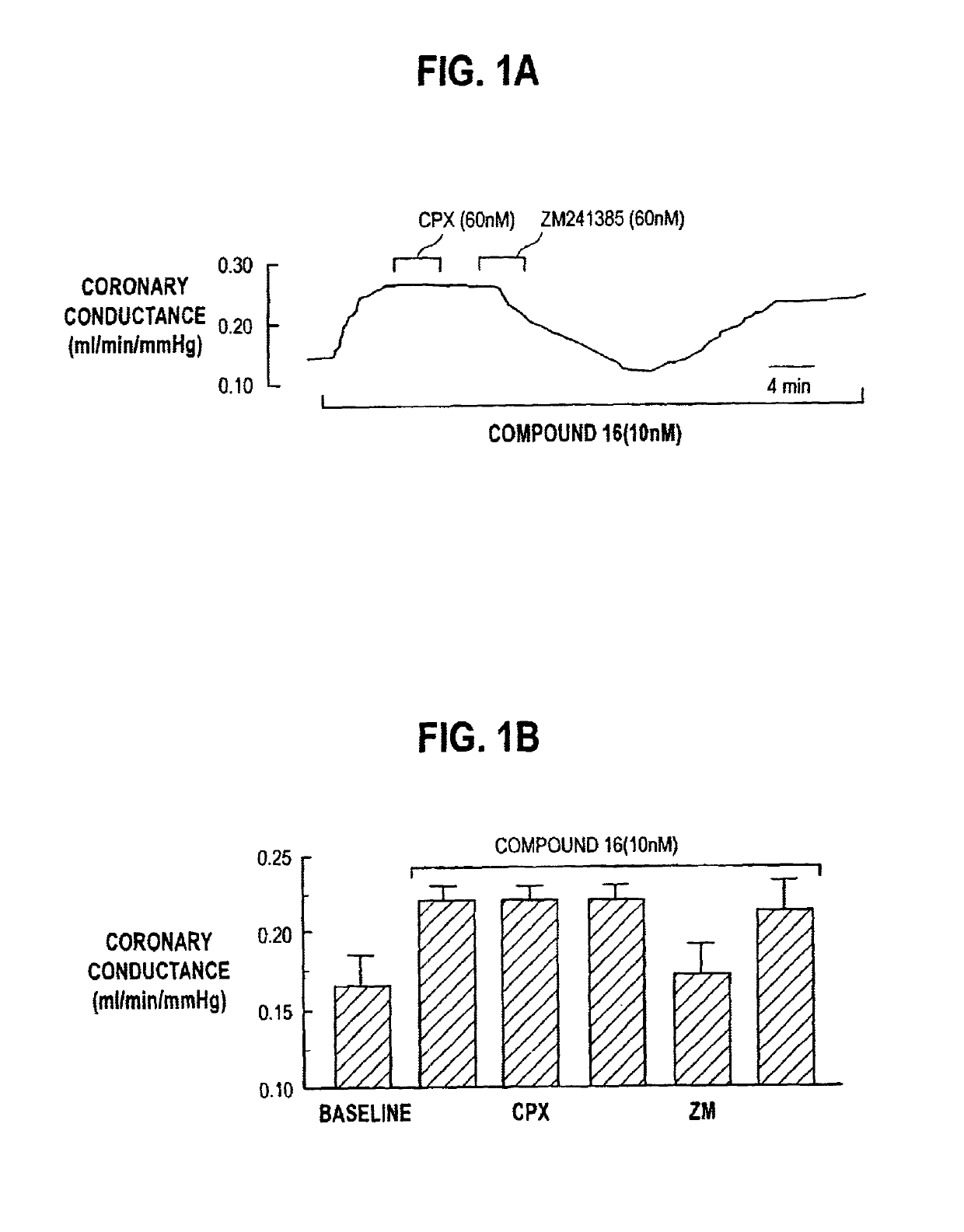
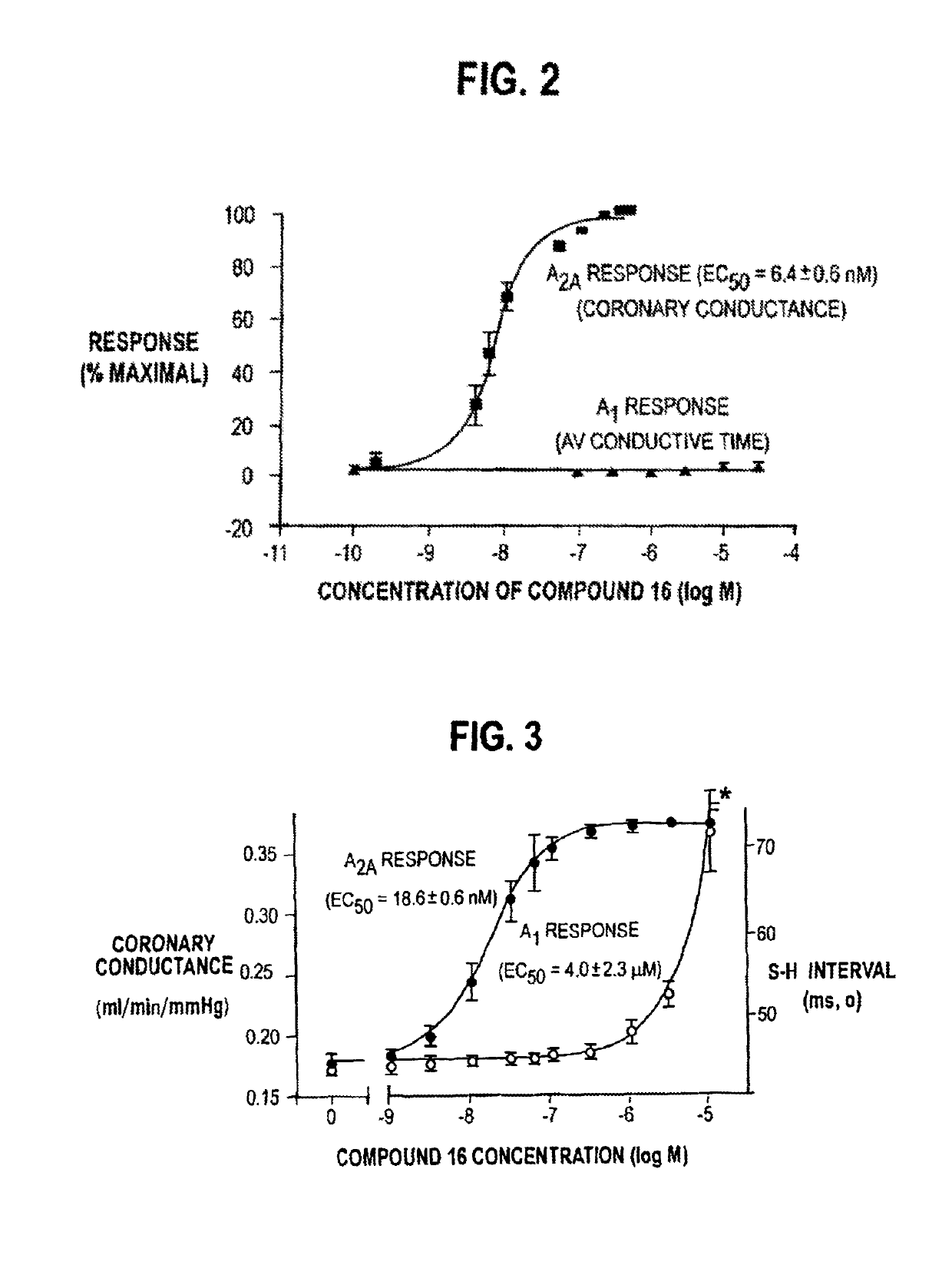
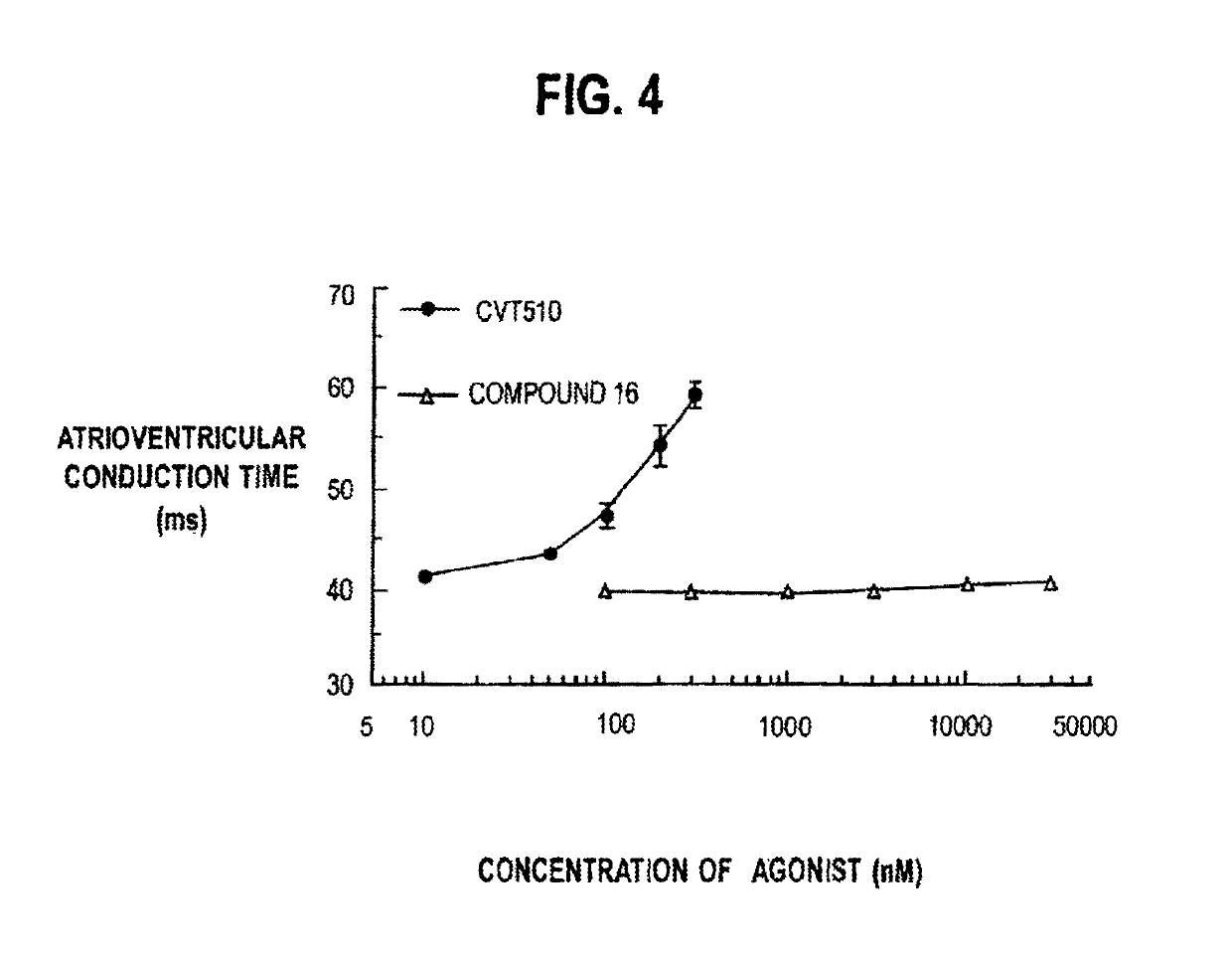
2-(N-pyrazolo)adenosines with application as adenosine A2A receptor agonists
a technology of adenosine receptor and adenosine a, which is applied in the field of npyrazole substituting 2adenosine compounds to achieve the effect of convenient coronary imaging and convenient us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0081]
Ethyl 1-{9-[(4S,2R,3R,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-aminopurin-2-yl}pyrazole-4-carboxylate (12)
[0082]To a suspension of 2-hydrazinoadenosine (0.025 g, 0.08 mmol) in a 1:1 mixture of MeOH / AcOH was added (ethoxycarbonyl)malondialdehyde (0.019 g, 0.12 mmol) and the mixture was heated at 80° C. for 3 h. The precipitate formed was collected by filtration and washed with EtOH and ether to afford 12. 1HNMR (DMSO-d6) δ1.25 (t, 3H), 3.5 (m, 1H), 3.6 (m, 1H), 3.8 (d, 1H), 4.15 (d, 1H), 4.55 (m, 1H), 5.0 (t, 1H), 5.2 (d, 1H), 5.5 (d, 1H), 5.5 (d, 1H), 5.9 (d, 1H), 7.15-7.3 (m, 5H), 7.8 (br s, 2H), 8.1 (s, 1H), 8.4 (s, 1H), 8,9 (s, 1H).
example 2
[0083]
(4S,2R,3R,5R)-2-{6-Amino-2-[4-(4-chlorophenyl)pyrazolyl]purin-9-yl}-5-(hydroxymethyl)oxolane-3,4-diol (13)
[0084]To a suspension of 2-hydrazinoadenosine (0.025 g, 0.08 mmol) in a 1:1 mixture of MeOH / AcOH was added 2-(4-chloro)phenylmalondialdehyde (0.022 g, 0.12 mmol) and the mixture was heated at 80° C. for 3 h. The precipitate formed was collected by filtration and washed with EtOH and Ether to afford 13. 1HNMR (DMSO-d6) δ3.5 (m, 1H), 3.6 (m, 1H), 3.8 (d, 1H), 4.15 (d, 1H), 4.2 (q, 2H), 4.55 (m, 1H), 5.9 (d, 1H), 7.45 (d, 2H), 7.75 (d, 2H), 8.25 (s, 1H), 8.35 (s, 1H), 8.9 (s, 1H).
example 3
[0085]
(4S,2R,3R,5R)-2-{6-Amino-2-[4-(4-metboxyphenyl)pyrazolyl]purin-9-yl}-5-(hydroxymethyl)oxolane-3,4-diol (14)
[0086]To a suspension of 2-hydrazinoadenosine (0.025 g, 0.08 mmol) in a 1:1 mixture of MeOH / AcOH was added 2-(4-methoxy)phenylmalondialdehyde (0.022 g, 0.12 mmol) and the mixture was heated at 80° C. for 3 h. The precipitate formed was collected by filtration and washed with EtOH and Ether to afford 14. 1HNMR (DMSO-d6) δ3.55 (m, 1H), 3.65 (m, 1H), 3.75 (s, 3H), 3.9 (d, 1H), 4.15 (d, 1H), 4.6 (m, 1H), 5.9 (d, 1H), 6.75 (d, 2H), 7.6 (d, 2H), 8.15 (s, 1H), 8.35 (s, 1H), 8.8 (s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electrical conductance | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com