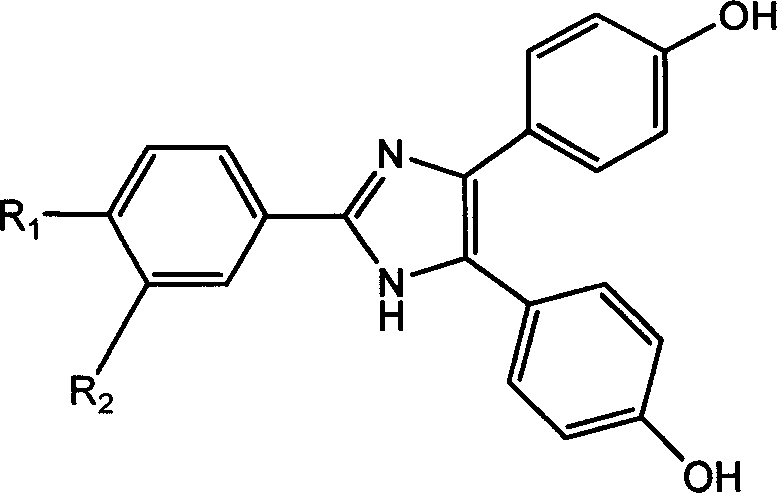
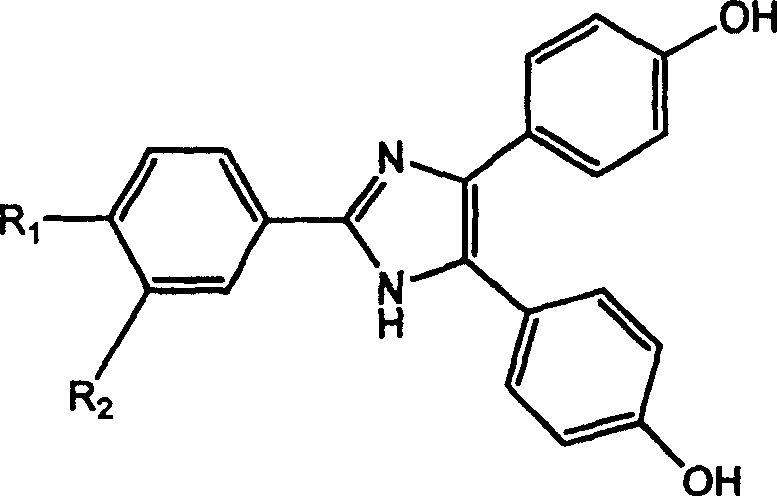
Type-Y second order non-linear optical chromophore containing imidazolyl heterocycle and its synthesis process
A second-order nonlinear and synthetic method technology, applied in the field of Y-type second-order nonlinear optical chromophore and its synthesis, can solve the problems of reducing molecular transparency, red-shifting molecular absorption wavelength, increasing β value, etc. Effects of improved thermal stability, large hyperpolarizability, and high thermal decomposition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
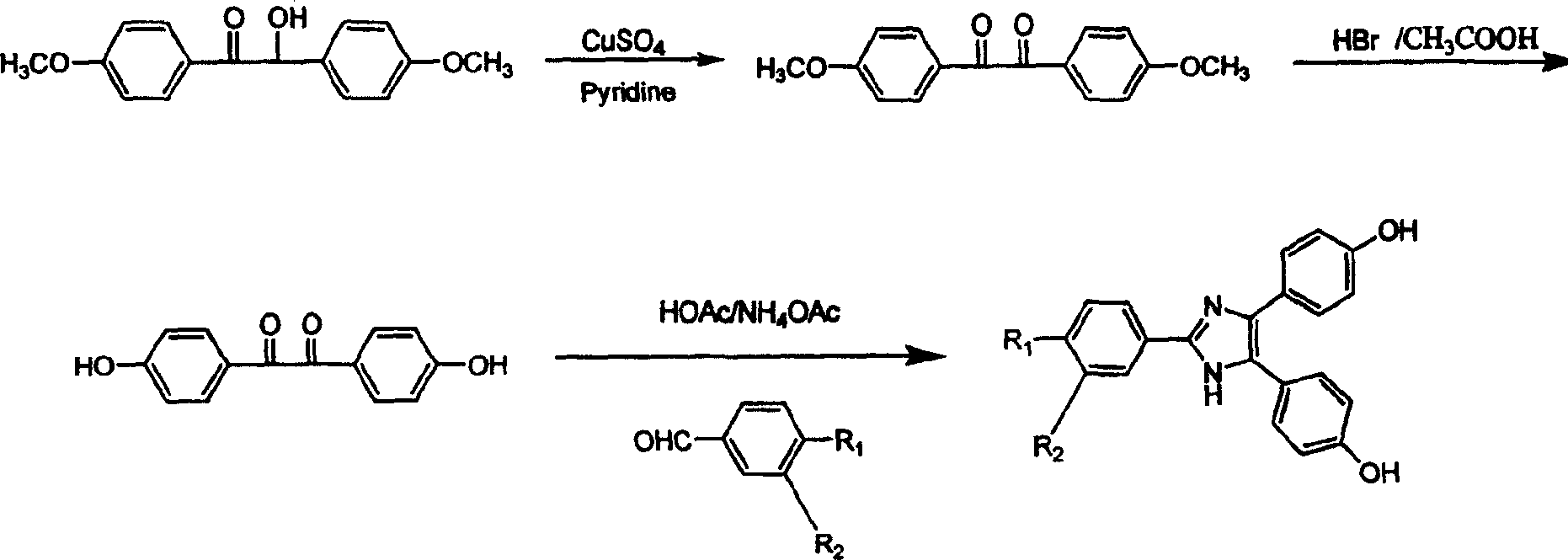
Embodiment 1
[0028] (1) Preparation of 1,2-bis-(p-methoxybenzene)-ethanone
[0029] Add 30 g of CuSO to a 100 ml round bottom flask 4 ·5H 2 O, 30ml of fresh pyridine, 9ml of deionized water, mixed and stirred, and heated to 40°C. Add 12g of 2-hydroxy-1,2-bis-(p-methoxybenzene)-ethanone, raise the temperature and continue stirring for 4-6 hours until the solution turns dark green, and pour it into deionized water to obtain a yellow-green product. Recrystallized from absolute ethanol to obtain 10.8 g of 1,2-bis-(p-methoxybenzene)-ethanone as yellow-green needle crystals with a yield of 90.7%.
[0030] (2) Preparation of 1,2-bis-(p-hydroxyphenyl)-ethanone
[0031] Under the situation of feeding nitrogen, 10g (0.037mol) of 1,2-di-(p-methoxybenzene)-ethanone and 100ml concentration of 48% hydrobromic acid were refluxed in 50ml glacial acetic acid and vigorously stirred for six After 1 hour, the solution was poured into water, and a fine gray powder precipitated out. The precipitate was diss...
Embodiment 2
[0042] (1) Preparation of 1,2-bis-(p-methoxybenzene)-ethanone
[0043] Add 30 g of CuSO to a 100 ml round bottom flask 4 ·5H 2 O, 30ml of fresh pyridine, 9ml of deionized water, mixed and stirred, and heated to 45°C. Add 12g of 2-hydroxy-1,2-bis-(p-methoxybenzene)-ethanone, raise the temperature and continue stirring for 4-6 hours until the solution turns dark green, and pour it into deionized water to obtain a yellow-green product. Recrystallized from absolute ethanol to obtain 10.8 g of 1,2-bis-(p-methoxybenzene)-ethanone as yellow-green needle crystals with a yield of 90.7%.
[0044] (2) Preparation of 1,2-bis-(p-hydroxyphenyl)-ethanone
[0045] Under the situation of feeding nitrogen, 10g (0.037mol) of 1,2-di-(p-methoxybenzene)-ethanone and 100ml concentration of 48% hydrobromic acid were refluxed in 50ml glacial acetic acid and vigorously stirred for six After 1 hour, the solution was poured into water, and a fine gray powder precipitated out. The precipitate was diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com