Compound for organic luminescent material and method for producing the same
A technology of light-emitting materials and compounds, applied in the fields of light-emitting materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of easy crystallization of materials, reduced light-emitting performance, and reduced light-emitting life of devices, and achieve the effect of good carrier transport performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
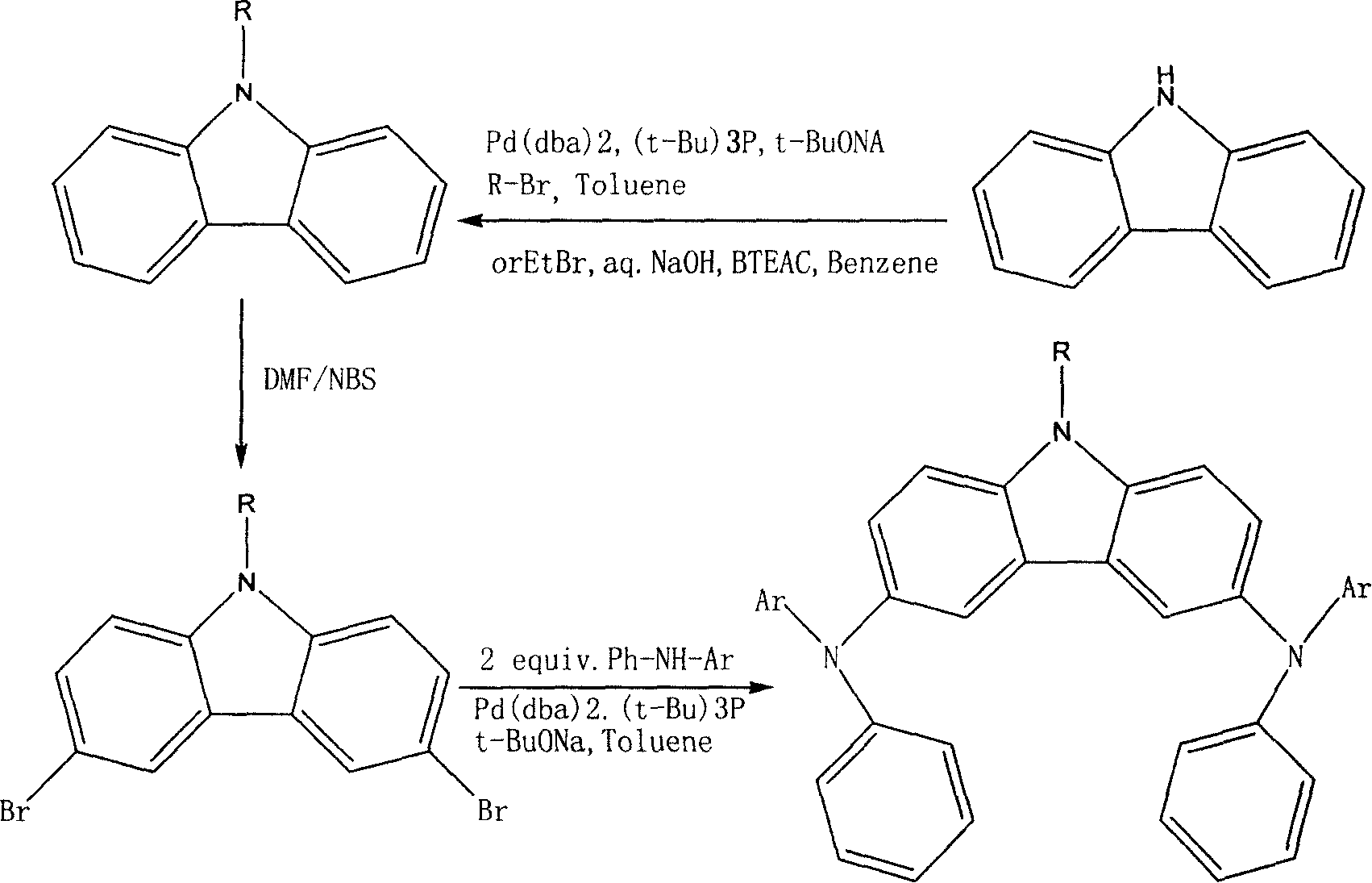
[0027] (1) Preparation of N-substituted carbazole:
[0028] Combine carbazole (1mmol, 0.167g), sodium tert-butoxide (1mmol, 0.96g), tert-butyl phosphine (0.02mmol, 0.0011g), bromoethane (1mmol, 0.11g), Pd(dba) 2 , (0.005mmol, 0.0028g) was added to a dry nitrogen-filled reaction system, 30ml xylene was added, heated to 100-120°C, and fully stirred to react for 2-3 hours. The temperature was lowered to room temperature, an appropriate amount of xylene was added for dilution, the organic layer was thoroughly washed with water, separated into layers, dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain the product.
[0029] (2) Preparation of N-substituted-3.6-dibromocarbazole:
[0030] Add NBS (2mmol, 0.356g), N'N-dimethylformamide (10mmol, 0.73g), and N-substituted carbazole (1mmol, 0.195g) into the reactor, and react at room temperature for three hours. Post-treatment: wash the reaction solution with brine, separate the layers, dry wit...
Embodiment 2
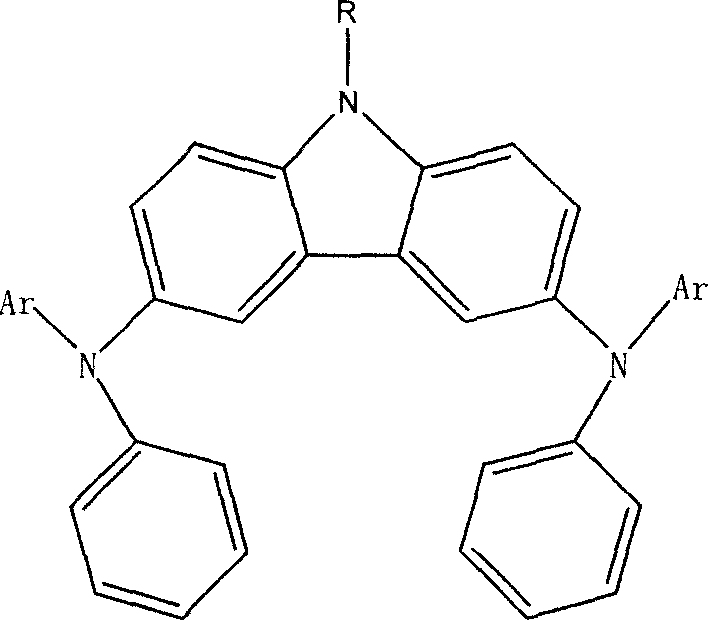
[0038] As in the other operations of Example 1, the bromoethane used in step (1) is replaced with bromobenzene. When the reaction temperature is 70°C, compound (2) is obtained. Its structural formula and physicochemical parameters are as follows:
[0039] 9-Phenyl-N,N’-dinaphthyl-N,N’-diphenyl-3,6-diaminocarbazole (2)
[0040]
[0041] Glass transition temperature: 123°C; absorption spectrum λmax=351nm;
[0042] Fluorescence spectrum λmax=487nm; solution fluorescence quantum yield Φ=0.07
Embodiment 3
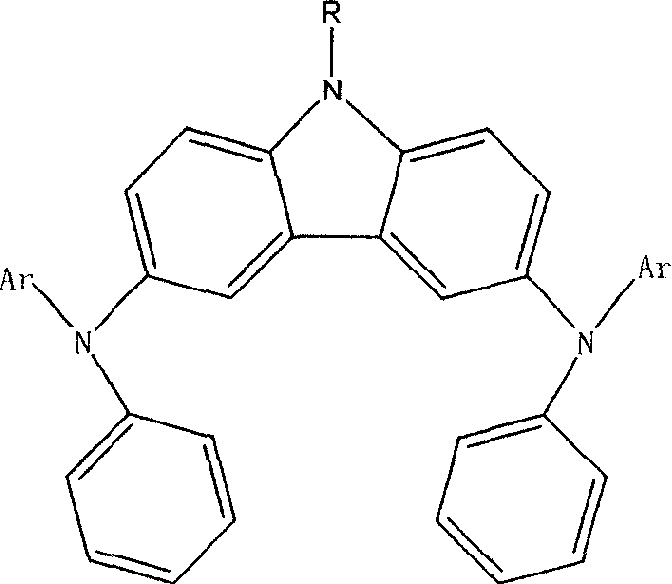
[0044] As in the other operations of Example 1, the phenylnaphthylamine used in step (3) was replaced with pyrenylphenylamine (0.586g, 2.0mmol), and the reaction temperature was 90℃ to obtain compound (3), structural formula and physicochemical The parameters are as follows:
[0045] 9-ethyl-N,N'-dipyrenyl-N,N'-diphenyl-3,6-diaminocarbazole (3)
[0046]
[0047] Glass transition temperature: 174℃; absorption spectrum λmax=408nm;
[0048] Fluorescence spectrum λmax=548nm; solution fluorescence quantum yield Φ=0.03
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com