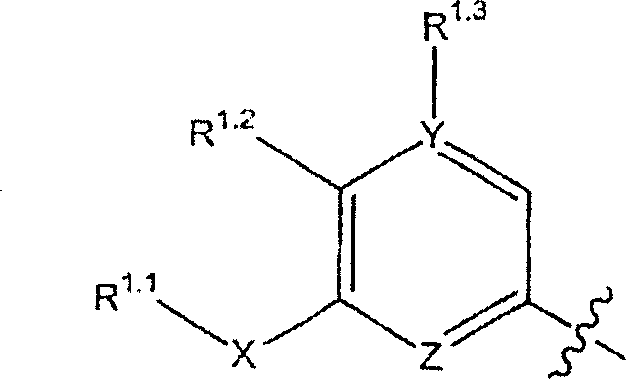
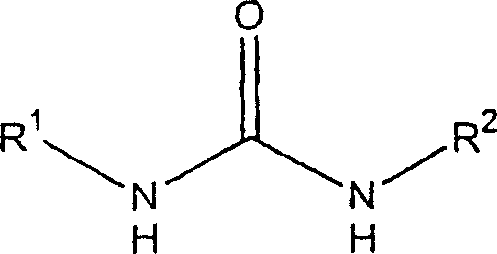
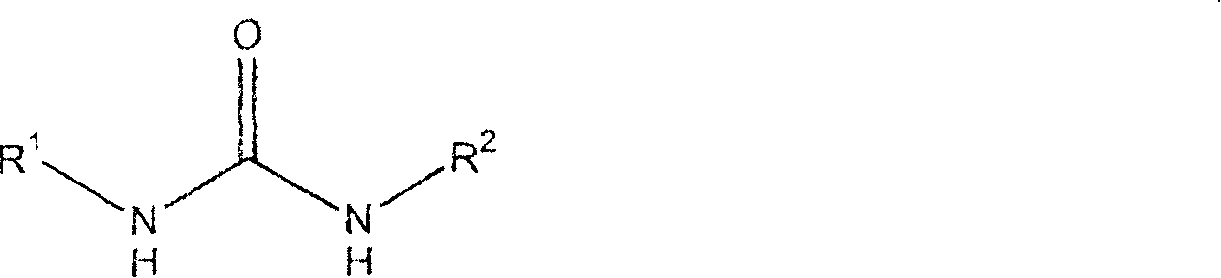Compounds, compositions and methods
A compound and mixture technology, applied in the field of pharmaceutical compositions, compounds for the treatment of systolic heart failure, and compounds for selectively regulating myocardial ganglion, can solve the problem of not meeting the gold standard of heart failure treatment, prolonging patient survival time, poor cardiac tissue, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0168] Scheme 3 shows the preparation of the compound of formula 305, which can be used as an intermediate in the synthesis of the compound of formula I.
[0169] Schemes 4 and 5 show the preparation of stereospecific reagents that can be used in the asymmetric synthesis of individual enantiomers of the compound of formula I.
[0170] Those skilled in the art will recognize that one or more of the reactants, steps and / or conditions described in these reaction routes can be adjusted to suit R 1 And R 2 Various substituents at the place.
[0171] material
[0172] Many of the optionally substituted starting compounds 101, 103, 201, 301a and 301b and other reagents are commercially available, for example from Aldrich Chemical Company (Milwaukee, WI), or by those skilled in the art through routine use The synthetic method is easily prepared.
[0173] Reaction route 1
[0174]
[0175] Preparation of compounds of formula I
[0176] Referring to reaction route 1...
Embodiment 1
[0446] (R)-1-[3-(1-Acetyl-piperidin-3-yloxy)-5-fluoro-phenyl]-3-pyridin-3-yl-urea
[0447] 1A. Preparation of SM 3
[0448]
[0449] (The amount shown below is the total amount used; therefore one-fourth of the total amount is added to each 50L flask)
[0450] Four 50L three-port RBF extractors equipped with a mechanical stirrer add 10.76kg (46.14mol) under nitrogen (IS)-(+)-Camphor-10-sulfonic acid (SM 2), 23.3 L (2.5 vol) ethanol (anhydrous), and 9.34 kg (92.27 mol) 3-hydroxypiperidine (SM 1). Add 142L of MTBE to make the solution cloudy. The solution was left overnight, then the solid was filtered out, and washed with 8L (1:1) MTBE: EtOH, 8L (2:1) MTBE: EtOH, and 8L MTBE to obtain 10.68 kg of white solid (35% yield, 75.8% ee, these values are the average of two batches). These solids were added to a 22L three-neck RBF flask equipped with a mechanical stirrer, thermometer, and reflux condenser. 10.7 L (1 vol) of ethanol (anhydrous) was added to the flask, and then the solut...
Embodiment 2
[0476] 3-{3-Fluoro-5-[3-(6-methyl-pyridin-3-yl)-ureido]-phenoxy}-pyrrolidine-1-sulfonic acid dimethylamide
[0477] 2A. (R)-tert-Butyl-3-(3-fluoro-5-nitrophenoxy)-pyrrolidine-1-carboxylate
[0478]
[0479] DMF (300 mL) and NaH (12.8 g, 320 mmol, 1.2 eq) were added to a round bottom flask, and then stirred in an ice bath. A solution of N-tert-butyl-(R)-3-hydroxypyrrolidine (50 g, 267 mmol, 1 eq) in DMF (100 mL) was slowly added to the flask, and then stirred for about 30 minutes. A solution of difluoronitrobenzene (51 g, 320 mmol, 1.2 eq) and DMF (50 mL) was added dropwise in about 30 minutes. The resulting solution was warmed to room temperature and stirred for about 4 hours. Water is added to the reaction mixture. The reaction solution was extracted with ethyl acetate. The organic layer was washed with water, saturated sodium bicarbonate, and brine solution. The organic layer was dried with sodium sulfate, filtered, and concentrated. The residue was purified by silica gel chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com