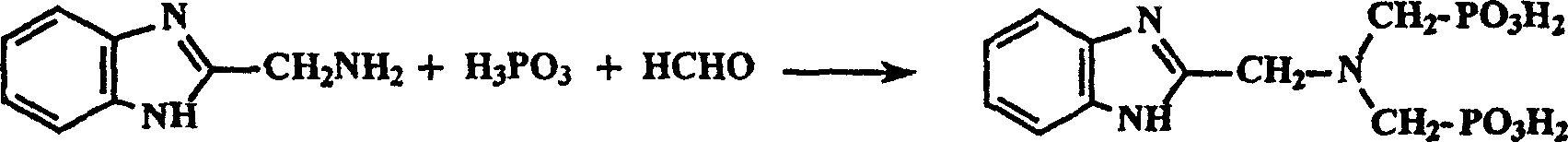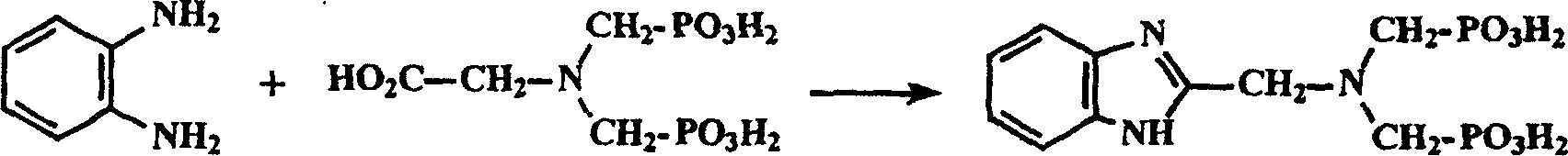N-(2-benzimidazolemethyl) imidodimethyl phosphonic acid synthesis method
A technology of iminobismethylphosphonic acid and benzimidazolylmethyl, which is applied in the field of synthesis of N-iminobismethylphosphonic acid [imino bis], can solve the problem of difficult purification and 2-benzimidazolylmethyl base) amine is more expensive, low yield and other problems, to achieve the effect of easy to obtain synthetic raw materials, cheap synthetic raw materials, and simple purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1.N-(2-benzimidazolyl methyl) iminobismethylphosphonic acid
[0025]
[0026] Mix N-(carboxymethyl) iminobismethyl sulfonic acid (20mmol, 5.26g) and o-phenylenediamine (20mmol, 2.16g) in a 100ml open round-bottomed flask, Heat in a bath. After about 2 hours, no more water vapor came out indicating the reaction was complete. Cool the reactant to about 100°C, add 10ml of 5mol·L -1 hydrochloric acid, and the mixture was refluxed in an oil bath at 120°C for 2 hours. The reaction solution was completely evaporated to dryness with a rotary evaporator, 20ml of distilled water was added thereto, KOH solid was added in batches until a clear solution with pH=5 was formed, activated carbon was added to reflux for 0.5 hours, and an orange-yellow clear solution was obtained by filtration after cooling. Slowly add 6mol L dropwise to the solution -1 hydrochloric acid with constant stirring until the pH of the mixture was ≤ 1.0, during which a large a...
Embodiment 2
[0028] The synthesis of embodiment 2.N-(2-benzimidazolyl methyl) iminobismethylphosphonic acid
[0029] Mix N-(carboxymethyl)iminobismethyl-acid (20mmol, 5.26g) and o-phenylenediamine (20mmol, 2.16g) in a 100ml open round-bottomed flask, Heat in a bath. After about 3 hours, no more water vapor came out indicating the reaction was complete. The reactant was cooled to about 100°C, and 8ml of 7mol·L -1 hydrochloric acid, and the mixture was refluxed in an oil bath at 120°C for 2 hours. The reaction solution was completely evaporated to dryness with a rotary evaporator, 16ml of distilled water was added thereto, KOH solid was added in batches until a clear solution with pH=6 was formed, activated carbon was added to reflux for 0.5 hours, and an orange-yellow clear solution was obtained by filtration after cooling. Slowly add 6mol.L dropwise to the solution -1 hydrochloric acid with constant stirring until the pH of the mixture was ≤ 1.0, during which a large amount of white pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com