Composition of Essentiale Phospholipids injection and preparation method thereof
A technology of polyene phosphatidylcholine and composition, applied in the field of pharmacy, can solve the problems of easy oxidative decomposition, side effects of bacteriostatic agents, poor stability, etc., and achieve the effects of improving compliance, stabilizing quality and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
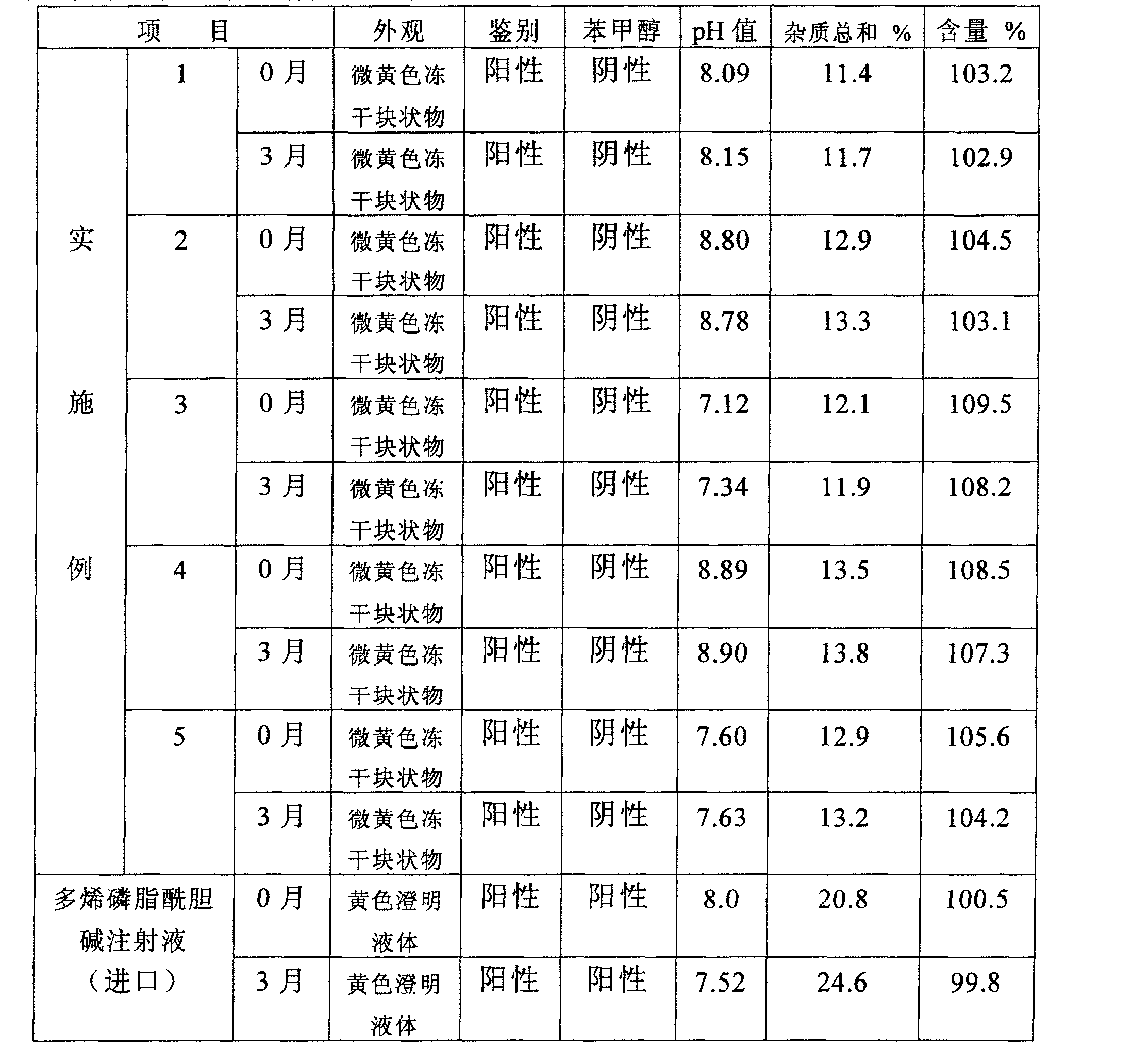
[0025] Under nitrogen and aseptic conditions, weigh 10.0 g of refined soybean lecithin, add a small amount of ethanol to dissolve, add 8.0 g of sodium cholate and 4.0 g of mannitol to dissolve, fully stir and dissolve to make a transparent solution, add, add 1 mol / L sodium hydroxide solution to adjust the pH value to 8.0, add water for injection to 100ml, mix well, filter through a 0.22μm microporous membrane, pack in vials, fill with nitrogen after freeze-drying, plug, and roll the cap to seal. , each bottle containing polyene phosphatidylcholine 200-1500mg white to yellowish powder injection.
Embodiment 2
[0027] Under nitrogen and aseptic conditions, weigh 10.0 g of refined soybean lecithin, add a small amount of methanol to dissolve, add 6.0 g of sodium deoxycholate, 3.0 g of glycine and water for injection, fully stir and dissolve to form a transparent solution, and adjust the pH value To 8.6, add water for injection to 100ml, mix well, filter through a 0.22μm microporous membrane, pack in vials, fill with nitrogen, stopper, cap, and seal after freeze-drying, and each bottle contains polyene Phosphatidylcholine 200-1500 mg white to slightly yellow powder injection.
Embodiment 3
[0029] Under nitrogen and aseptic conditions, weigh 10.0 g of refined soybean lecithin, dissolve in ethanol, add 4.5 g of sodium glycocholate, 6.0 g of lactose and water for injection, fully stir and dissolve to form a transparent solution, and adjust the pH value to 7.0, add water for injection to 100ml, mix well, filter through a 0.22μm microporous membrane, pack in vials, fill with nitrogen, stopper, cap, and seal after freeze-drying, and each bottle contains polyene phospholipids. Acylcholine 200-1500 mg white to slightly yellow powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com