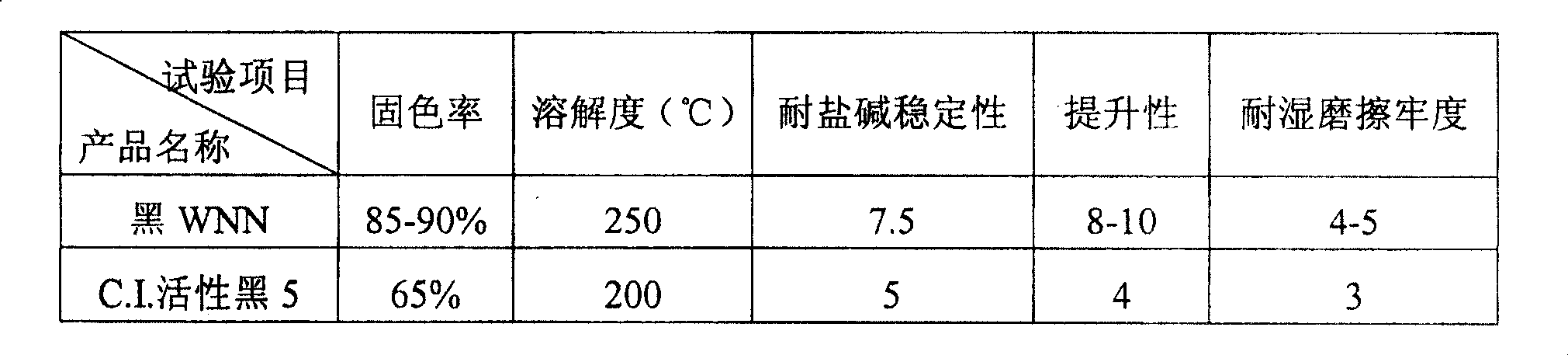
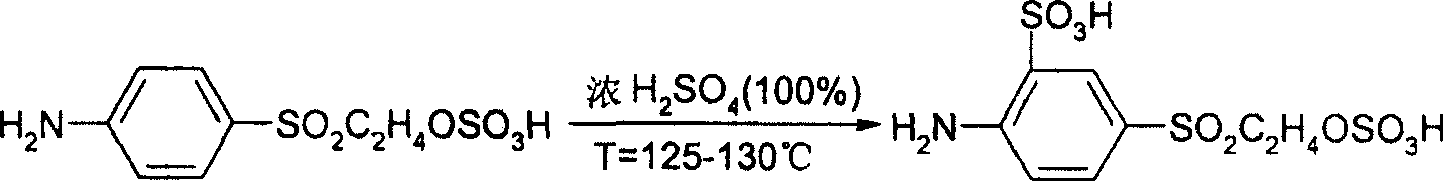Preparation method of para-(beta-Ethyl Sulfonyl Sulfate) aniline sulfonic acid
A kind of technology of sulfate ethyl sulfone and aniline ortho, applied in the field of preparation of intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Use concentrated sulfuric acid, p-(β-sulfate ethyl sulfone) aniline to add dilute hydrochloric acid to prepare intermediate p-(β-sulfate ethyl sulfone) aniline o-sulfonic acid, the steps are as follows:
[0022] 1. Disperse, add 1000kg of concentrated sulfuric acid (100%) into the reaction pot, slowly add 696kg of p-(β-sulfate ethyl sulfone) aniline (100%) under constant stirring, control the temperature at 80-85°C, beat After 2 hours, check the dispersion of p-(β-sulfate ethyl sulfone) aniline to make it exist without lumps;
[0023] 2. Sulfonation, turn on the steam and slowly heat up to 125-130°C, and react at this temperature for 8 hours, and detect by high performance liquid chromatography (HPLC), if p-(β-sulfate ethyl sulfone) aniline≤0.5% , then the sulfonation is in place, such as p-(β-sulfate ethyl sulfone) aniline>0.5%, then continue the insulation reaction until p-(β-sulfate ethyl sulfone) aniline≤0.5%.
[0024] 3. Hydrolysis, after cooling the...
Embodiment 2
[0026] Embodiment 2: Use concentrated sulfuric acid, p-(β-sulfate ethyl sulfone) aniline to add dilute sulfuric acid to prepare intermediate p-(β-sulfate ethyl sulfone) aniline o-sulfonic acid, the steps are as follows:
[0027] 1. Disperse, add 1000kg of concentrated sulfuric acid (100%) into the reaction pot, slowly add 696kg of p-(β-sulfate ethyl sulfone) aniline (100%) under constant stirring, control the temperature at 80-85°C, beat After 2 hours, check the dispersion of p-(β-sulfate ethyl sulfone) aniline, if there is no block, proceed to the next step;
[0028] 2. Turn on the steam and slowly heat up to 125-130°C, and react at this temperature for 8 hours, detect with high performance liquid chromatography (HPLC), if p-(β-sulfate ethyl sulfone) aniline≤0.5%, then The sulfonation is in place; if p-(β-sulfate ethylsulfone) aniline>0.5%, the heat preservation reaction should be continued until p-(β-sulfate ethylsulfone)aniline≤0.5% is qualified;
[0029] 3. After cooling ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com