Method for preparing pharmaceutical pure quetiapine fumarate
A technology for preparing quetiapine fumarate and drugs, applied in organic chemistry and other fields, can solve environmental pollution and other problems, achieve the effects of reducing costs, solving serious environmental pollution, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] ① Chlorination: Add 20Kg of dibenzo[b.f][1.4]thiazepine-11-[10H]ketone, 128Kg of toluene, 6.5Kg of N-N-dimethylaniline, and 28Kg of phosphorus oxychloride to the reaction In the tank, through N 2 , stir and heat up to reflux, time the reaction for 6.5 hours, when the reaction is over, cool down to 17°C, add ice (slowly) 100Kg, stir for 30 minutes, filter, extract by layer, wash with water until neutral, dehydrate under reduced pressure, decolorize, and get chlorine compound in toluene.
[0029] ② Addition into salt: put the toluene liquid of the above chloride into the reaction tank according to 20Kg ring compound, N-[2-(2-hydroxyethoxy) ethyl]piperazine 10Kg, and anhydrous sodium carbonate 12Kg , stir and heat up to reflux, time the reaction for 6.5 hours, when the reaction is over, filter, add 5% hydrochloric acid 40Kg layered extraction, then add solid Na 2 CO 3 Adjust the pH to 10-11, add 55Kg of toluene to dissolve, wash with water until neutral, and dehydrate u...
Embodiment 2
[0032] 1. Chlorination: the process steps are the same as in Example 1, except that some technical parameters are different: add 120Kg of toluene, 7.2Kg of N-N-dimethylaniline, 40Kg of thionyl chloride, timed reaction for 7 hours, cool to 20°C, stir for 20 minute.
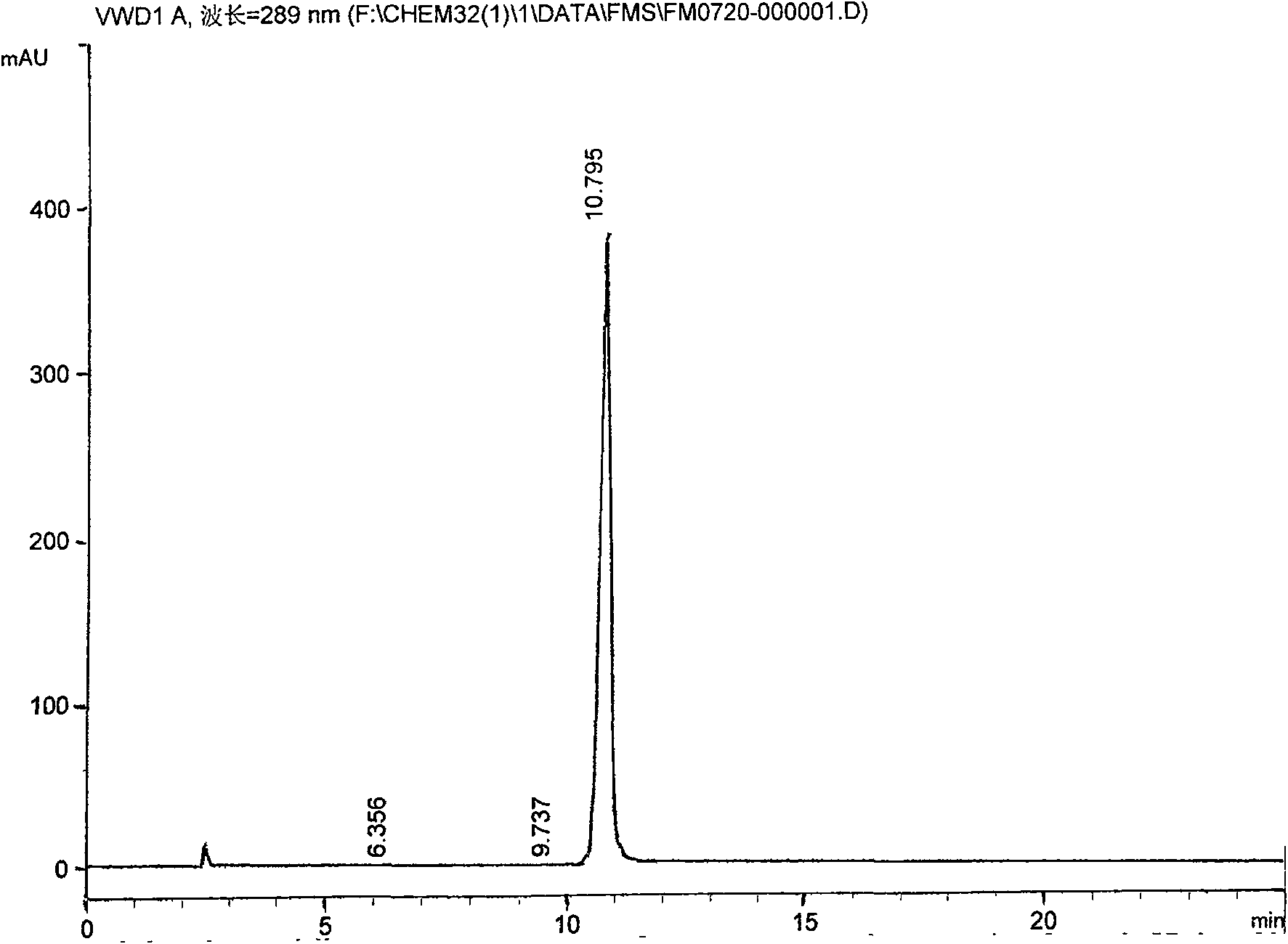
[0033] 2. Addition into salt: process step is the same as embodiment 1, but some technical parameters are different: add N-[2-(2-hydroxyethoxy) ethyl] piperazine 13Kg, anhydrous sodium carbonate 11Kg, timed reaction 6 hours , add 7% hydrochloric acid, adjust pH9-10, get quetiapine 24Kg, add 4Kg of fumaric acid to get quetiapine fumarate 24.0Kg, addition salt yield 67%, relevant impurity content 0.085%. Its spectrum is as figure 2 As shown, the peak area table is as follows:
[0034]
Embodiment 3
[0036] 1. Chlorination: the process steps are the same as in Example 1, except that some technical parameters are different: add 130Kg of toluene, 7Kg of N-N-dimethylaniline, 39Kg of phosphorus oxychloride, timed reaction for 7 hours, cool to 20°C, and stir for 30 minutes .
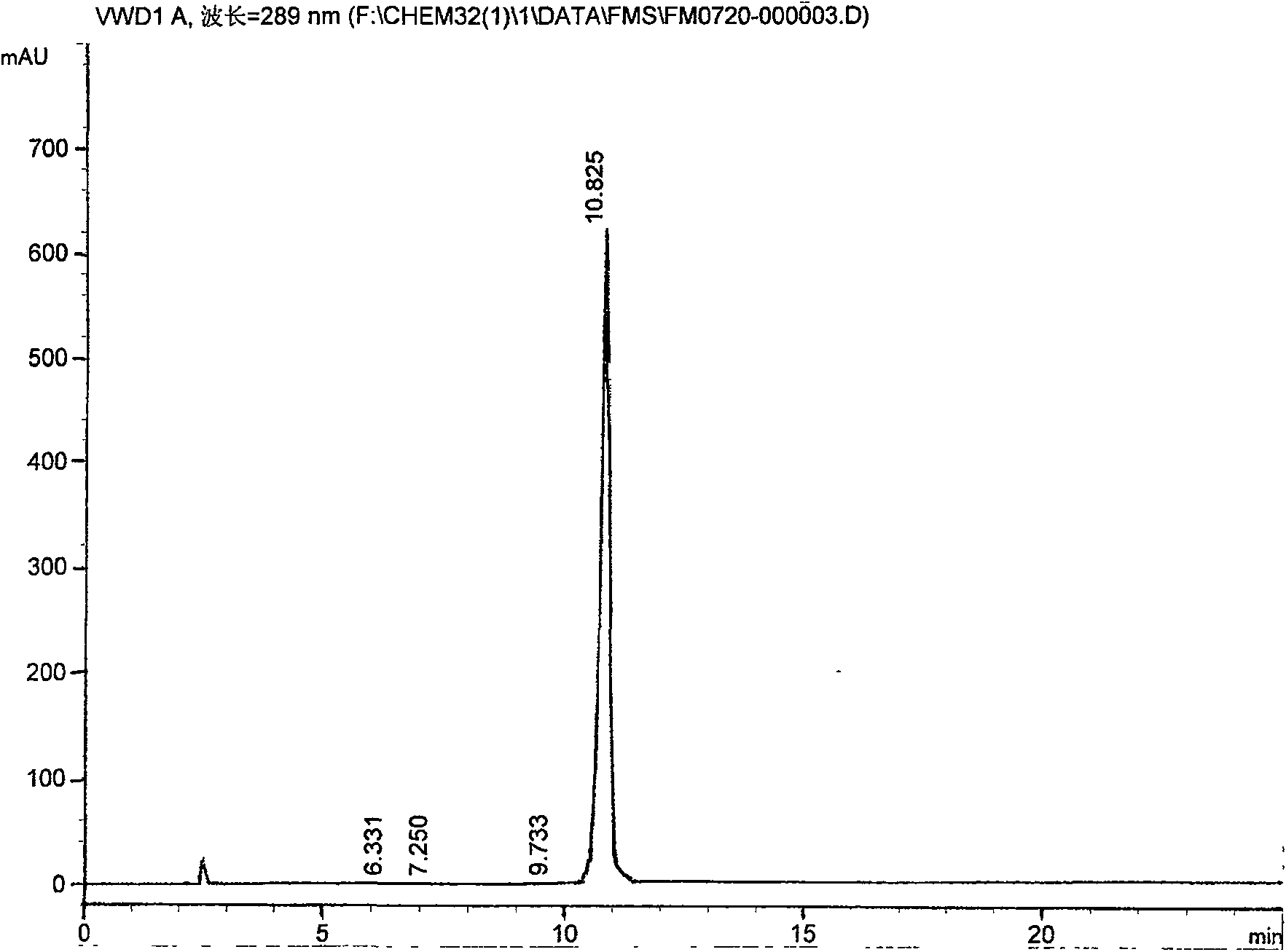
[0037] 2. Addition into salt: process step is the same as embodiment 1, but some technical parameters are different: add N-[2-(2-hydroxyethoxy) ethyl] piperazine 14Kg, anhydrous sodium carbonate 13Kg, timed reaction 6.5 hours , add 7% hydrochloric acid, adjust PH10-11, get quetiapine 24.0Kg, add 3.8Kg fumaric acid, get quetiapine fumarate 24.3Kg, one-way yield 67.8%, relevant impurity content 0.075%. Its spectrum is as image 3 As shown, the peak area table is as follows:
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com