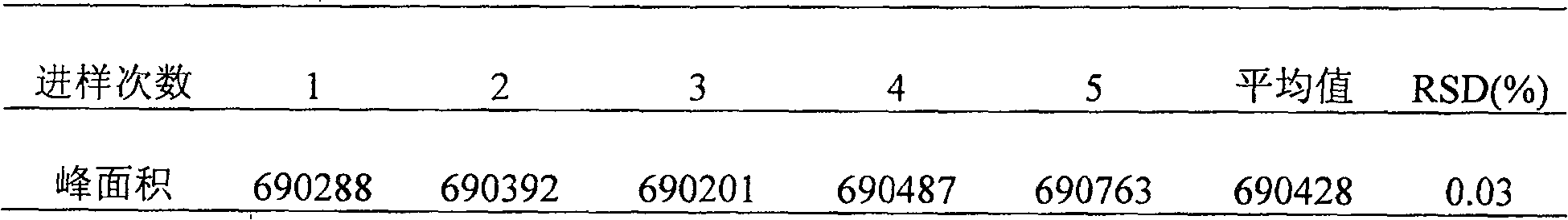Quality control method for Chinese medicine preparation
A cold-clearing heat-clearing and detection method technology, which is applied to medical formulas, medical preparations containing active ingredients, plant raw materials, etc., can solve problems such as ineffective control of the quality of cold-clearing heat preparations, affecting product production and quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] The quality control of embodiment 1 Ganmao Qingre syrup
[0117] The quality control method of the present invention comprises the following steps:
[0118] The observation of traits, the steps are:
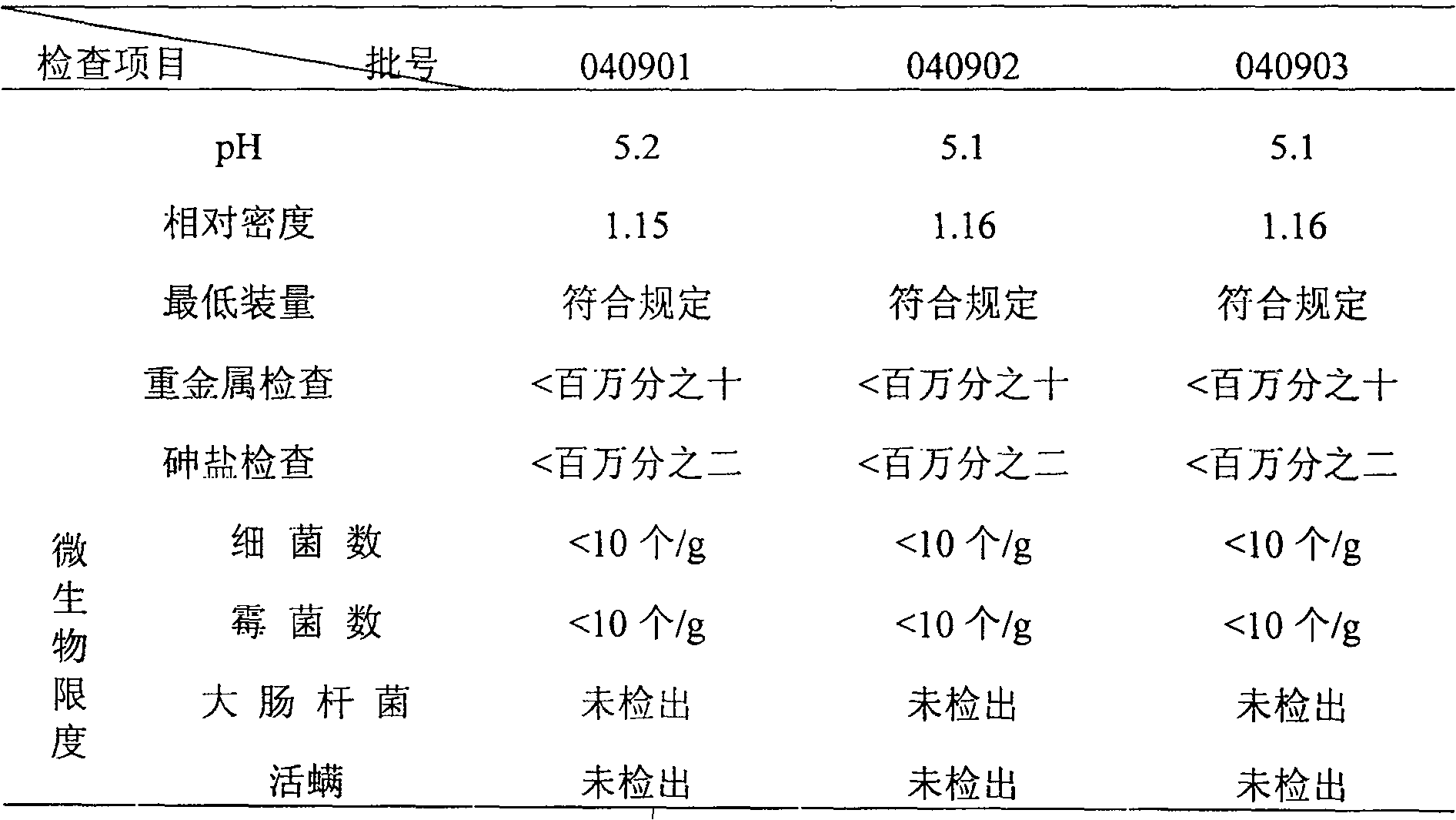
[0119] 【Properties】This product is a brownish-yellow to brown liquid; sweet, slightly bitter.
[0120] Content identification, the steps are:
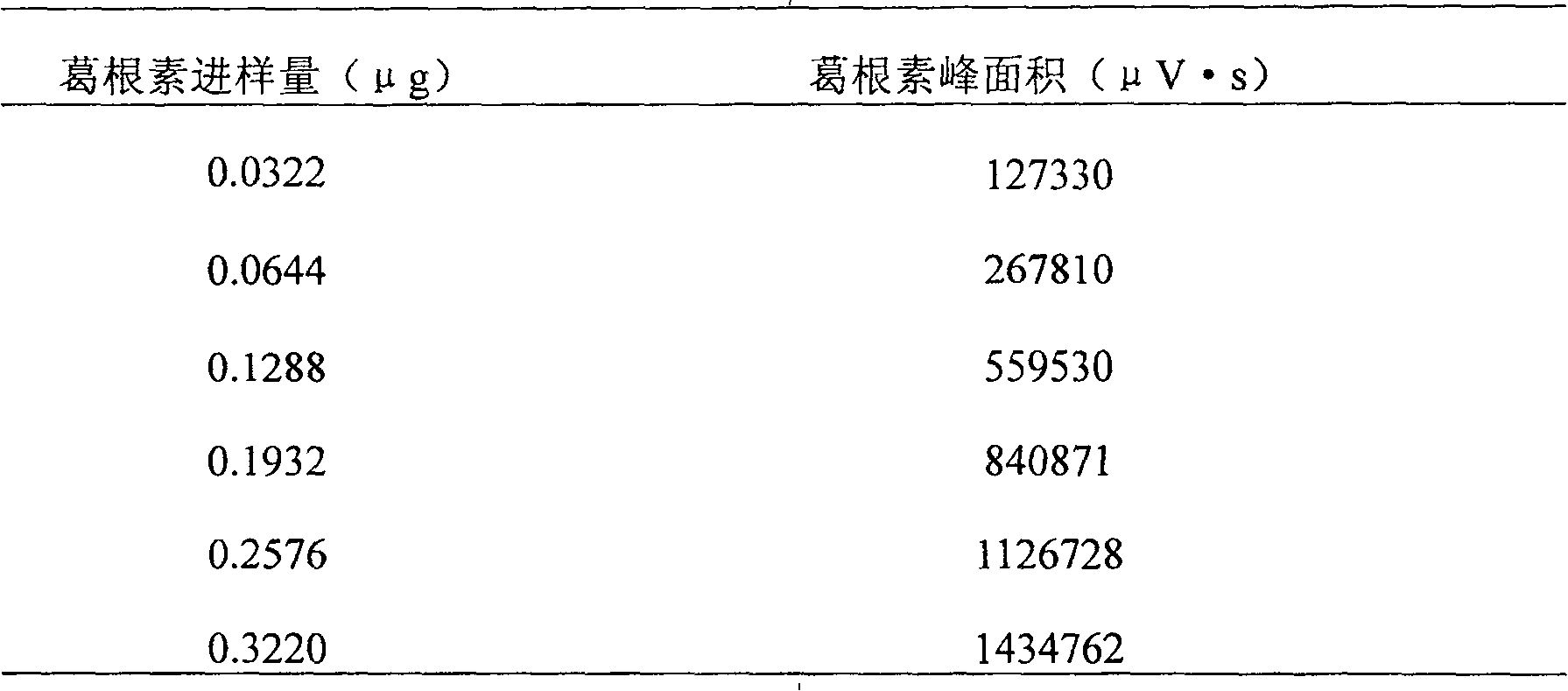
[0121] [Identification] (1) Take 15ml of this product, dilute with 5ml of water, add ethyl acetate and shake and extract twice, 20ml each time, combine the ethyl acetate solution, evaporate to dryness, add 0.5ml of methanol to dissolve the residue, and use it as the test sample solution. Take another puerarin reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. According to the test of thin-layer chromatography (Appendix VI B, Chinese Pharmacopoeia, 2005 edition), draw 5 μl of each of the above two solutions, respectively spot on the same silica gel G thin-layer plate...
Embodiment 2
[0137] The quality control of embodiment 2 Ganmao Qingre Oral Liquid
[0138] The quality control method of the present invention comprises the following steps:
[0139] The observation of traits, the steps are:
[0140] 【Properties】This product is brown to brown translucent liquid; sweet, slightly bitter.
[0141] Content identification, the steps are:
[0142] [Identification] (1) Take 15ml of this product, dilute with 5ml of water, add ethyl acetate and shake and extract twice, 20ml each time, combine the ethyl acetate solution, evaporate to dryness, add 0.5ml of methanol to dissolve the residue, and use it as the test sample solution. Take another puerarin reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. According to the test of thin-layer chromatography (Appendix VI B, Chinese Pharmacopoeia, 2005 edition), draw 5 μl of each of the above two solutions, respectively spot on the same silica gel G thin-layer...
Embodiment 3
[0158] Example 3 Quality Control of Ganmao Qingre Granules
[0159] The quality control method of the present invention comprises the following steps:
[0160] The observation of traits, the steps are:
[0161] 【Properties】This product is granules, brown to brown powder or granules; sweet, slightly bitter.
[0162] Content identification, the steps are:
[0163][Identification] (1) Take 15g of this product, dilute with 5ml of water, add ethyl acetate and shake and extract twice, 20ml each time, combine the ethyl acetate solution, evaporate to dryness, add 0.5ml of methanol to dissolve the residue, and use it as the test sample solution. Take another puerarin reference substance, add methanol to make a solution containing 1mg per 1ml, as the reference substance solution. According to the test of thin-layer chromatography (Appendix VI B, Chinese Pharmacopoeia, 2005 edition), draw 5 μl of each of the above two solutions, respectively spot on the same silica gel G thin-layer p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com