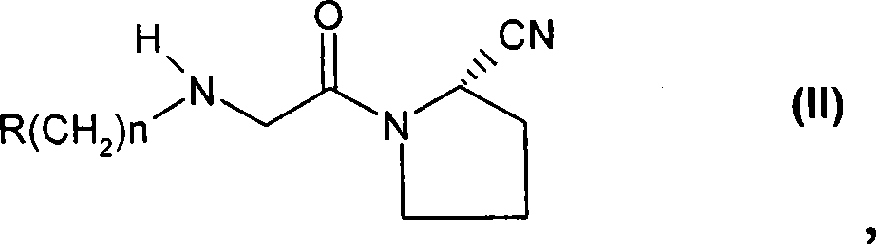
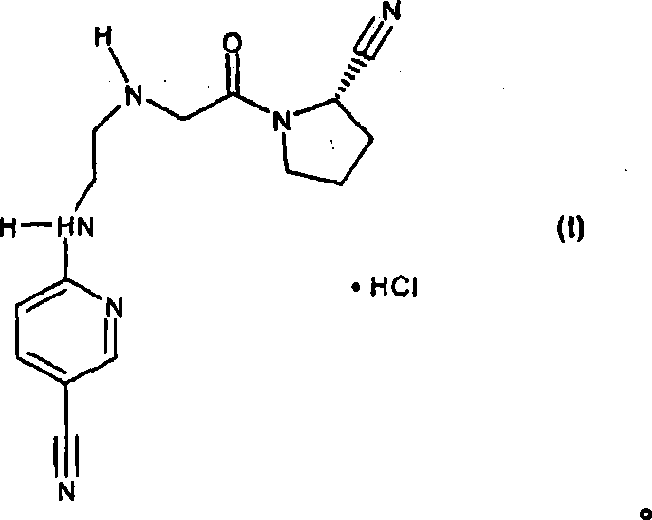
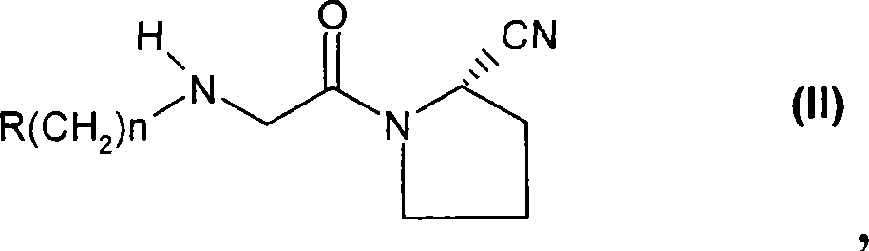
Fast release composition including melt granules of a moisture sensitive drug and process for manufacturing thereof
A composition and sensitive technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, metabolic diseases, etc., can solve problems such as chemical instability and difficulty in making pharmaceutically acceptable oral compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Solid oral dosage forms prepared by dry mixing
[0096] Compound I was first passed through a 25 mesh screen and 11.2 g were obtained. Compound I and 100 g of lactose were placed in a 1 quart V-blender and tumbled for 5 minutes. Transfer the mixture to a 25 mesh screen and return to the V-blender. The talc, crospovidone and remaining lactose were then added to the V-blender and the mixer was inverted for an additional 10 minutes. Separately, pass the hydrogenated castor oil through a 60 mesh screen. The hydrogenated castor oil was then added to the V-blender and tumbled for 5 minutes. The blend was then compressed on a Manesty B3B tablet press using round, standard concave and beveled edge dies. To prevent sticking, polish the mold beforehand. The obtained 150 mg tablet contains about 5 mg of Compound I and is designated herein as "Sample 1". Sample 1 contained no Compound I particles coated or substantially coated with hydrogenated castor oil.
Embodiment 2
[0098] Solid oral dosage form prepared from melt-granulated granules using hydrogenated castor oil
[0099] As a comparison with Sample 1, a solid oral dosage form was prepared from melt-granulated Compound I. Compound I and the hydrophobic melt component, hydrogenated castor oil, were passed through 25 mesh and 60 mesh sieves, respectively. These ingredients were then added to the 1 L pan of a Key International (Englishtown, NJ) Model KG5 high shear granulator.
[0100] A heating mantle surrounds the pan and sets the rheostat at 80°C. This granulator is equipped with an impeller and no chips. Turn on the impeller to mix the therapeutic compound and hydrophobic melt components.
[0101] After mixing, the granules were removed from the pan and spread onto aluminum foil to cool. These granules were then sieved with a Frewitt shaker.
[0102] These granules were then transferred to a V-blender filled with microcrystalline cellulose and crospovidone. The V-blender was turned...
Embodiment 3
[0106] Solid oral dosage form prepared from melt-granulated granules using stearic acid
[0107] As a comparison with Sample 1 and Sample 2, another solid oral dosage form was prepared from melt-granulated granules of Compound I. The same procedure as disclosed in Example 2 was used; however, all of the hydrogenated castor oil was replaced with stearic acid. Thus, stearic acid is a hydrophobic melt component in the melt-granulated granules and a lubricant for the tablet itself. These tablets are designated herein as "Sample 3".
[0108] Table 1 summarizes the compositions of samples prepared in Examples 1, 2, and 3.
[0109] Table 1
[0110]
sample
sample 1
(mg)
sample 2
(mg)
sample 3
(mg)
Compound I
Hydrogenated castor oil as melt component
Stearic acid as molten component
Spray-dried lactose as a bulking agent
Crospovidone as a disintegrant
Talc as an anti-sticking agent
Hydrogenated castor oil as a lubr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com