Complex containing mequitazine, a cyclodextrin and an interaction agent
A technology of mequitazine and complexes, applied in medical preparations containing active ingredients, medical preparations with non-active ingredients, drug combinations, etc., can solve the problems of low solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0093] Example 2: Preparation of mequitazine / β-cyclodextrin / arginine complex
[0094] The procedure was the same as that in Example 1 except for the change of reagent dosage. 2.5 grams of mequitazine in racemic and / or D- or L-form, 20.11 grams of β-cyclodextrin, 1.35 grams of arginine, and 5.63 grams of water were used.
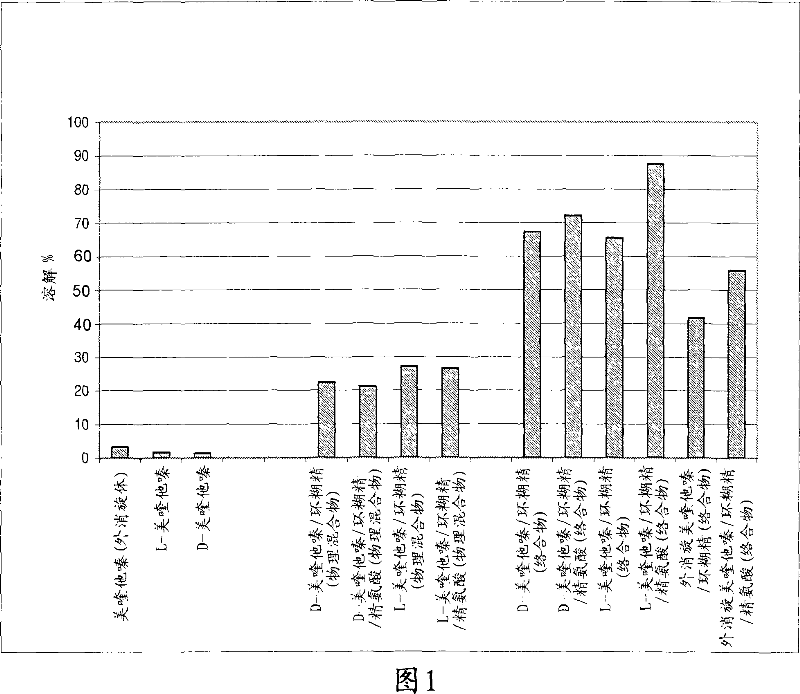
[0095] Solubility and dissolution ratios of complexed mequitazine (D, L or racemate) were determined as indicated in "Mequitazine Dissolution Test". The results are listed in Table 1 and shown in Figure 1 below.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com