Recombination human acidic mechanocyte growth factor temperature sensitive type gel preparation and preparation method thereof
A technology of fibroblasts and growth factors, applied in medical preparations with no active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of haFGF gel formula and preparation process that have not been seen, and achieve Reduce the pain of medication, easy to clean, the effect of a reasonable matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription:
[0021] aFGF 4.5×10 6 IU
[0022] Heparin sodium 1.5×10 6 IU
[0023] Poloxamer 407 200g
[0024] Glycerin 50g
[0025] Ethanol 50g
[0026] Ethyl p-hydroxybenzoate 2g
[0027] Water for injection up to 1000g
[0028] Preparation:
[0029] Take 800ml water for injection, add 200g poloxamer while stirring, fully swell to form a solution at 4°C, add 50g glycerin, 50g ethanol and 2g ethyl p-hydroxybenzoate, mix well, and then under the conditions of 0.1Mpa and 121°C Autoclave for 15 minutes, cool to 4℃, make the matrix part, mix rh-aFGF (4.5×106IU) solution and heparin sodium (1.5×106IU) in a ratio of 3:1, filter and sterilize, add to the sterile Add sterile water for injection to 1000 g in the post-bacterial gel matrix, slowly stir and mix uniformly to prepare rh-aFGF temperature-sensitive gel.
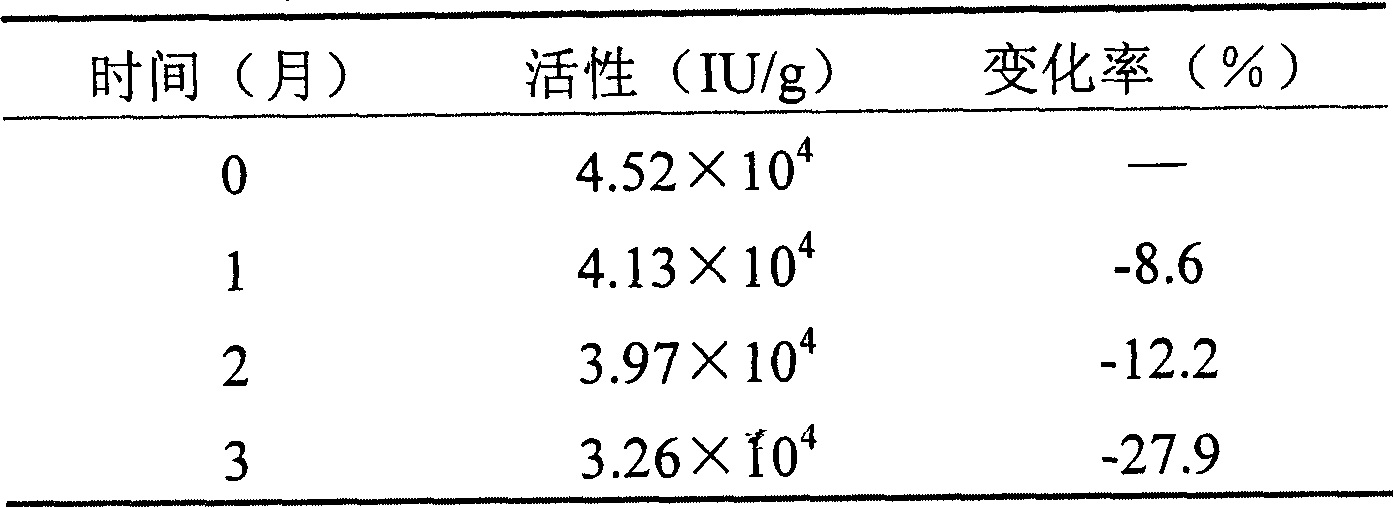
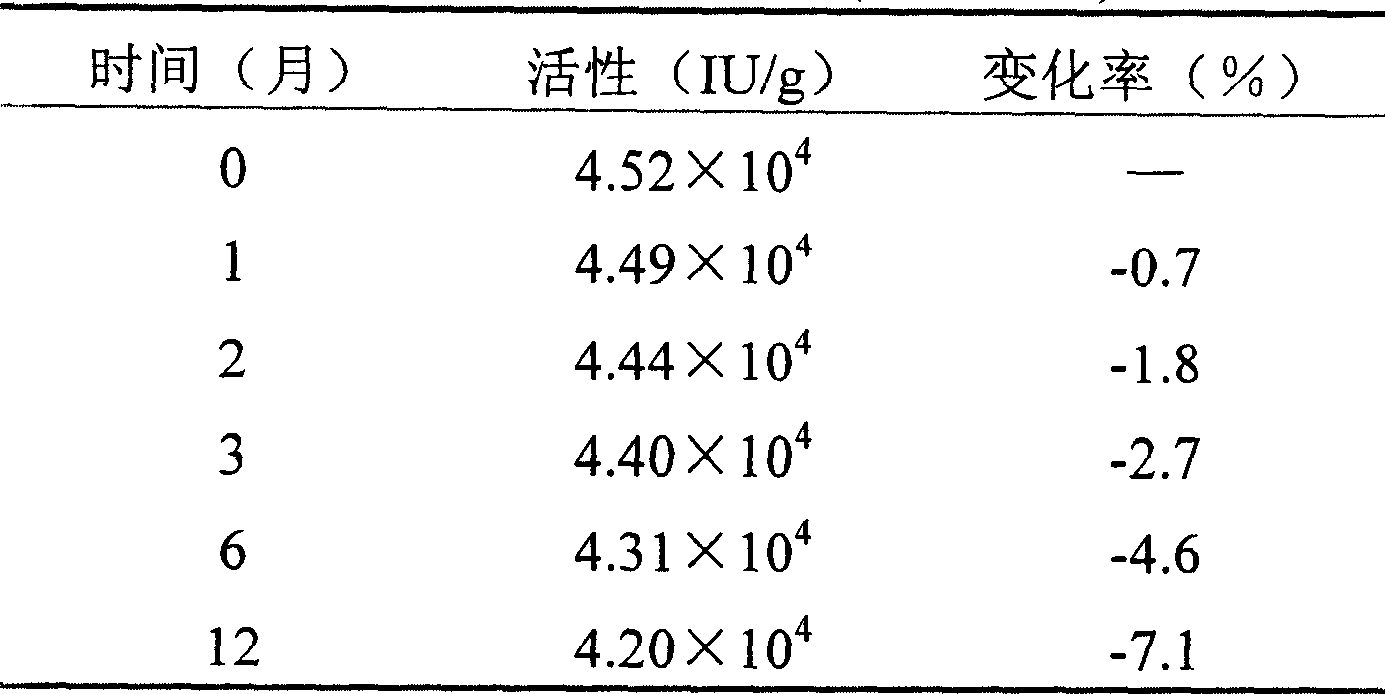
[0030] Stability test of rh-aFGF temperature-sensitive gel:
[0031] Prepare rh-aFGF temperature-sensitive gel according to Example 1. Take an appropriate amount of the gel and ...
Embodiment 2
[0041] prescription:
[0042] aFGF 3×10 6 IU
[0043] Heparin Sodium 1×10 6 IU
[0044] Poloxamer 407 280g
[0045] Glycerin 70g
[0046] Propylene glycol 30g
[0047] Yamanashi 5g
[0048] Water for injection up to 1000g
[0049] Take 800ml water for injection, add 280g poloxamer while stirring, fully swell at 4℃ to form a solution, add 70g glycerol, 30g propylene glycol and 5g sorbic acid, mix well, then autoclave at 0.1Mpa, 121℃ 15 Minutes, cool to 4°C, make the matrix part, add rh-aFGF(3×10 6 IU) solution and heparin sodium (1×10 6 IU) Mix well at a ratio of 3:1, filter and sterilize, add to the sterilized gel matrix, add sterilized water for injection to 1000g, slowly stir and mix evenly to make rh-aFGF temperature-sensitive gel Agent.
Embodiment 3
[0051] prescription:
[0052] aFGF 5.7×10 6 IU
[0053] Heparin Sodium 1.9×10 6 IU
[0054] Poloxamer 407 300g
[0055] Dextran 2.5g
[0056] Ethanol 20g
[0057] Sodium benzoate 1g
[0058] Water for injection up to 1000g
[0059] Take 800ml water for injection, add 300g poloxamer while stirring, fully swell to form a solution at 4℃, add 80g glycerol, 20g ethanol and 1g sodium benzoate, mix well, then autoclave at 0.1Mpa, 121℃ 15 After cooling to 4℃, make the matrix part, take 2.5g of dextran and dissolve it with appropriate amount of water for injection, add rh-aFGF (5.7×10 6 IU) and heparin sodium (1.9×10 6 IU) Mix the solution at a ratio of 3:1 and mix it evenly, filter and sterilize and add it to the sterilized gel matrix, add sterilized water for injection to 1000g, slowly stir and mix well to make rh-aFGF temperature sensitive Type gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com