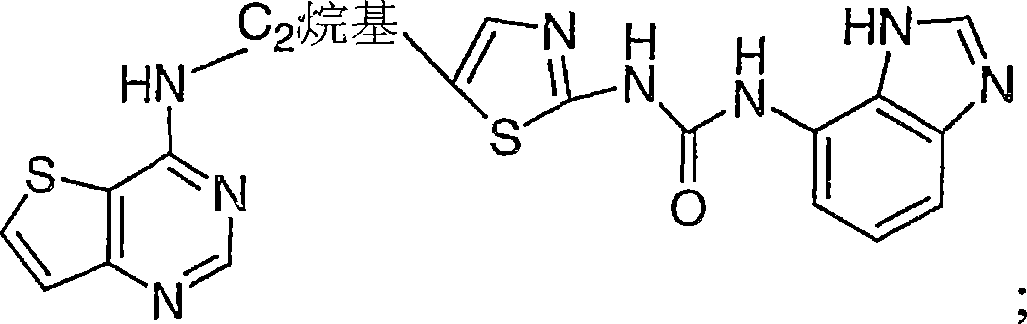
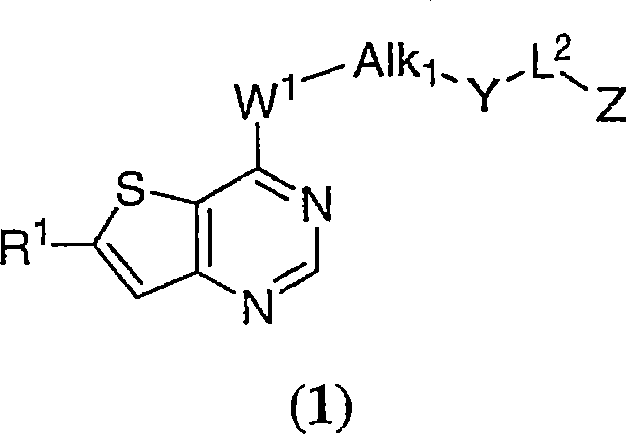
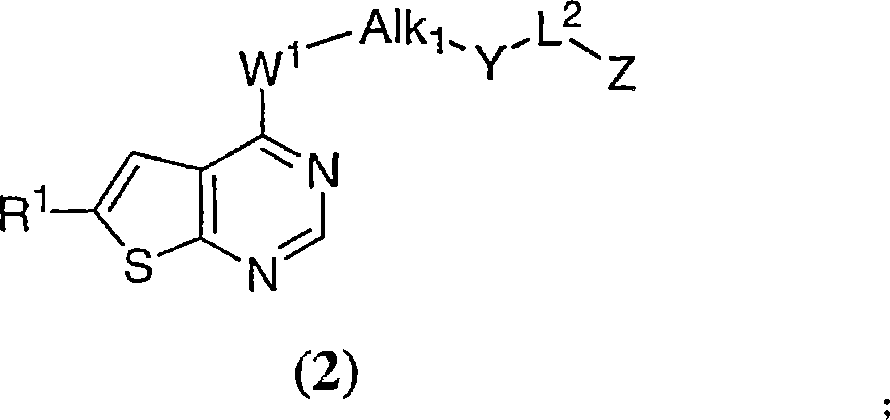
Thienopyrimidines useful as aurora kinase inhibitors
A technology of CR1AR1B and compounds, applied in the field of thienopyrimidines suitable for use as AURORA kinase inhibitors, can solve problems such as insufficient treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0649] This example describes the synthesis of:
[0650]
[0651] Step 1: [2-(4-Amino-phenyl)-ethyl]-carbamic acid tert-butyl ester (compound 1.1; 1.0 mmol, according to Hah , J.M. et al., prepared by the procedure of J.Med.Chem 46, 2003, 1661) and triethylamine ("TEA"; 3.0 equivalents) in anhydrous tetrahydrofuran ("THF"; 5.0 mL). After the reaction was complete, the mixture was partitioned between water and ether. The organic layer was separated, washed with 1.0N HCl, saturated sodium bicarbonate and brine and dried. Purification by flash column chromatography on silica gel gave [2-(4-benzoylamino-phenyl)-ethyl]-carbamic acid tert-butyl ester (compound 1.2).
[0652] Step 2: Compound 1.2 (1.0 mmol) was treated with anhydrous 4.0 N HCl in dioxane (25 mL) at 0°C, stirred at room temperature for 2 hours and concentrated to dryness under reduced pressure. The crude amine salt 4-chloro-7-methylthieno[3,2-d]pyrimidine (1.0 equiv) and N,N-diisopropylethylamine ("DIEA"; 2.5 eq...
example 2
[0654] This example describes the synthesis of:
[0655]
[0656] where R 1 is as previously described. These compounds were prepared according to the procedure of Example 1 except that in Step 2 Instead of 4-chloro-7-methylthieno[3,2-d]pyrimidine. R 1 Illustrative examples of are found throughout this disclosure and in Table 1.
[0657] Table 1
[0658]
[0659]
example 3
[0661] This example describes the synthesis of:
[0662]
[0663] where R 1 is as previously described. These compounds were prepared according to the procedure of Example 1, except that in Step 2 Instead of 4-chloro-7-methylthieno[3,2-d]pyrimidine. R 1 Illustrative examples of are found throughout this disclosure and in Table 2.
[0664] Table 2
[0665]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com