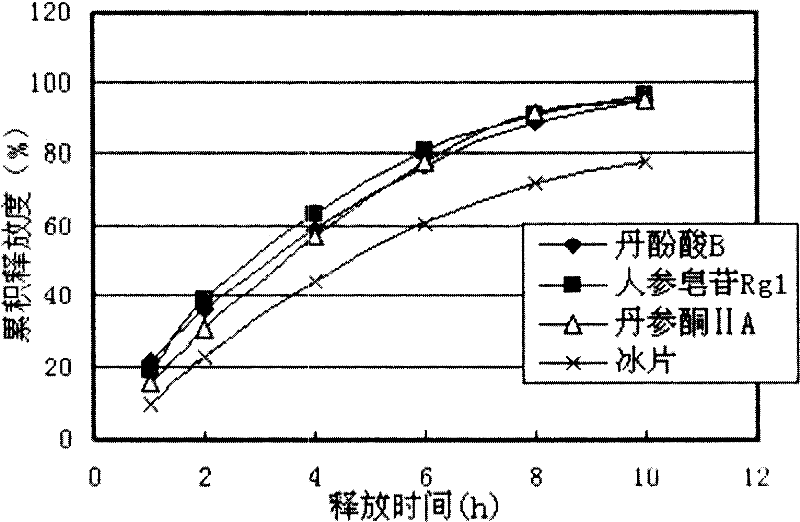
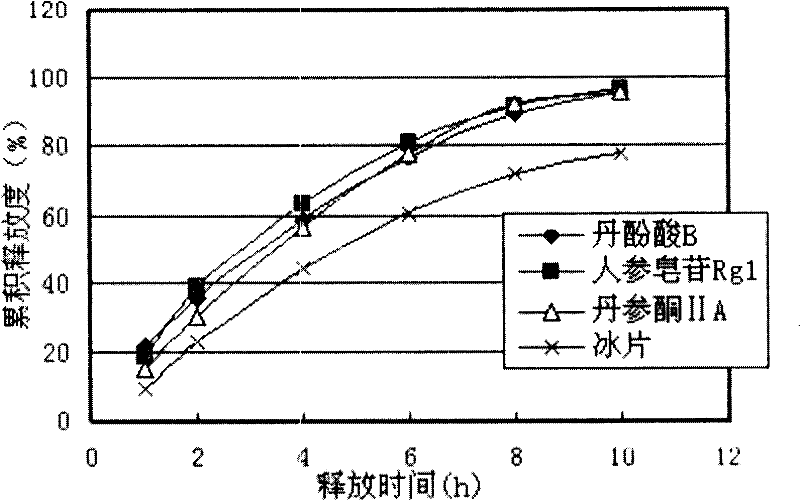
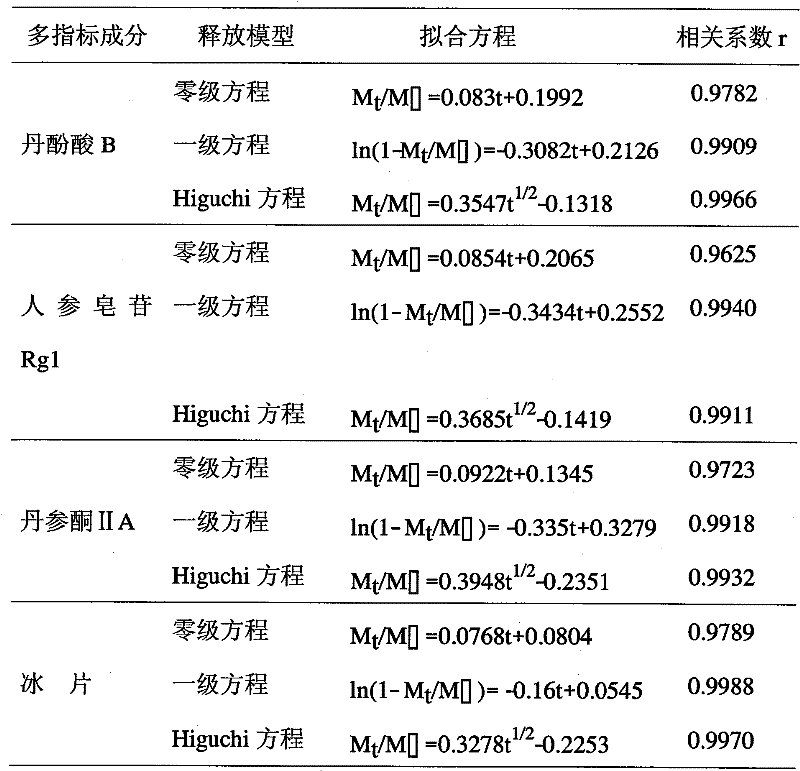
Compound red sage root extended release formulation and preparation thereof
A slow-release preparation, the technology of compound salvia miltiorrhiza, applied in the direction of medical formula, medical preparations containing active ingredients, etc., can solve the problem of balanced release of complex ingredients, to maintain effective blood drug concentration, delayed release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Sustained Release Tablets
[0032] (1) Embedding of borneol
[0033] Weigh 15g of natural beeswax in a water bath, heat and melt, add 15g of borneol, stir to fuse together, cool to below 10°C, crush and pass through a 20-mesh sieve to obtain 30g of borneol-embedded material;
[0034] (2) Preparation of sustained-release preparations
[0035] Weigh 15g of Salvia miltiorrhiza extract, 20g of Panax notoginseng extract, 50g of ethyl cellulose, mix well, add 18ml of 50% ethanol, granulate, dry, add 30g of borneol embedding material, 1g of magnesium stearate, mix well, and press into Compound salvia miltiorrhiza sustained-release preparation;
[0036] (3) Stomach and enteric film coating
[0037] Weigh 50g of acrylic resin I, soak it in 1000mL of 75% ethanol for 8 hours, dissolve it, add 15g of triethyl citrate, 15g of Tween 80, and 20g of titanium dioxide, stir well, pass through a 80-mesh sieve after being ground by a colloid mill. The compound ...
Embodiment 2
[0038] Example 2 Preparation of sustained-release capsules
[0039] (1) Embedding of borneol
[0040] Weigh 60g of natural beeswax in a water bath to heat and melt, add 40g of borneol, stir to fuse together, cool to below 10°C, crush and pass through a 20-mesh sieve to obtain 100g of borneol-embedded matter;
[0041] (2) Preparation of sustained-release preparations
[0042] Weigh 30g of Danshen extract, 15g of Panax notoginseng extract, 15g of ethyl cellulose, mix well, add 15ml of 50% ethanol, granulate, dry, granulate, add 1g of magnesium stearate, 100g of borneol embedding, measure Content, calculate filling volume, fill capsules.
Embodiment 3
[0043] Example 3 Preparation of Sustained Release Pills
[0044] (1) Embedding of borneol
[0045] Weigh 90g of natural beeswax in a water bath, heat and melt, add 10g of borneol, stir to fuse together, cool to below 10°C, crush and pass through a 20-mesh sieve to obtain 100g of borneol-embedded matter;
[0046] (2) Preparation of sustained-release preparations
[0047] Weigh 20g of Salvia miltiorrhiza extract, 10g of Panax notoginseng extract, 100g of borneol embedding material, 60g of hydroxypropyl methylcellulose, mix well, add 12g of PVP-k30, mix well, and directly press into sustained-release pills.
[0048] (3) Sustained-release film coating
[0049] Weigh 60g of ethyl cellulose, soak it in 500mL of 75% ethanol for 5 hours, dissolve it, add 10g of castor oil, 15g of lauryl sulfates, and 15g of polyethylene glycol, stir well, pass through a 80-mesh sieve after being ground by a colloid mill. The compound salvia miltiorrhiza preparation is spray-coated with a slow-release...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com