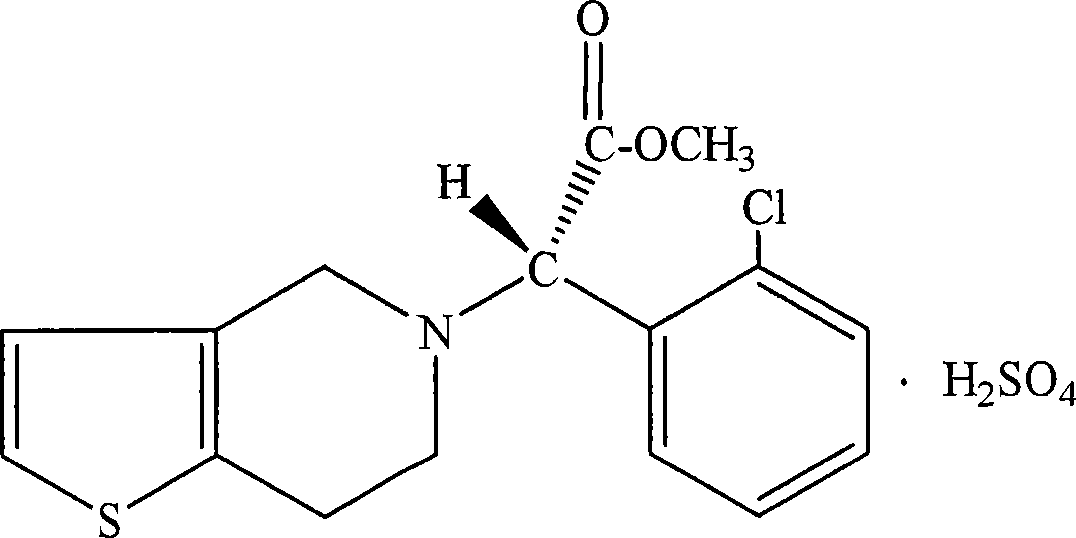
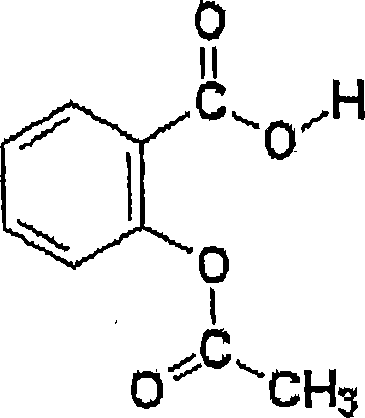
Nanoparticulate clopidogrel and aspirin combination formulations
A technology of granular clopidogrel and clopidogrel, applied in the field of nanoparticle clopidogrel and aspirin combined preparation, can solve problems such as difficult bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] The purpose of this example is to illustrate how to prepare the nanoparticulate clopidogrel / aspirin composition.
[0185] Aqueous dispersions of clopidogrel bisulfate can be combined with one or more surface stabilizers, followed by incorporation in NanoMill(R) 0.01 (NanoMill Systems, King of Prussia, PA; see eg US Pat. No. 6,431,478) for milling in a 10 ml chamber. The composition may be milled at a speed of 2500 for a suitable period of time, such as about 60 minutes.
[0186] The milled composition can be harvested and analyzed via microscopy. For example, Lecia DM5 000B and Lecia CTR 5000 light sources (Laboratory Instruments and Supplies Ltd., Ashbourne Co., Meath, Ireland) can be used for microscopy. Microscopic examination may reveal the presence of discrete clopidogrel nanoparticles.
[0187] The particle size of crushed clopidogrel microparticles was also measured in Milli Q Water with a Horiba LA-910 Particle Sizer (ParticularScience, Hatton Derbyshire, Eng...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com