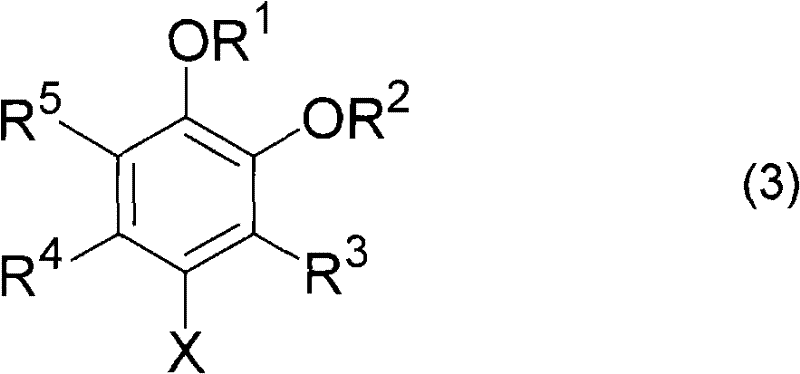
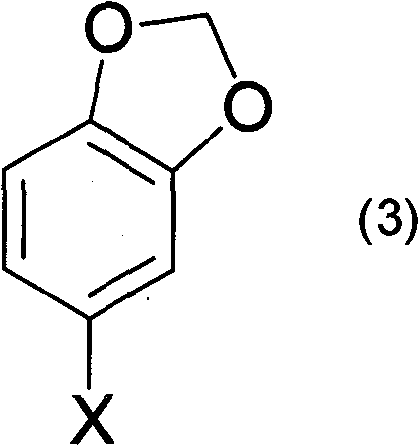
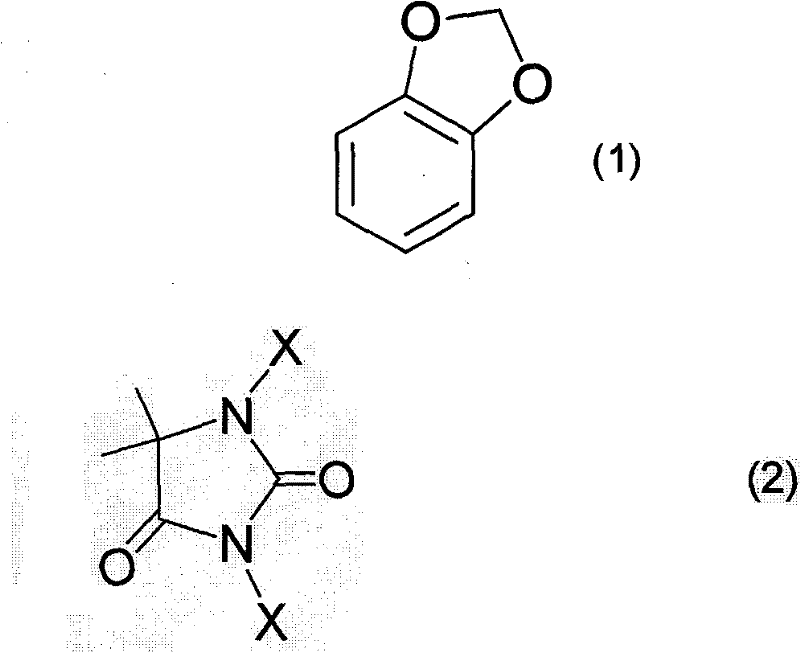
Preparation method of 4-halogenated catechol compound
A technology of halomethylenedioxybenzene and dihalogenation, which is applied in the field of high-purity 4-chloromethylenedioxybenzene and its preparation, and can solve the problems that do not mention 4-chloromethylenedioxybenzene Benzene purity and other issues, to achieve the effect of a simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1 (the synthesis of 4-chloromethylenedioxybenzene)
[0056] Into a glass flask with a capacity of 1000 ml equipped with a stirring device, a thermometer and a reflux condenser, under an argon atmosphere, 244.2 g (2.0 mol) of methylenedioxybenzene and 122 g of acetic acid were added, and the temperature of the liquid was raised to 55°C. Then, keeping the liquid temperature at 60-90°C, 216.7 g (1.1 mol) of 1,3-dichloro-5,5-dimethylhydantoin was slowly added, followed by reaction at 70°C with stirring for 1 hour. After the reaction, the reaction solution was cooled to room temperature, 245 ml of hexane and 245 ml of water were added to the reaction solution, stirred, and the organic layer was separated. The obtained organic layer was washed with a 10% aqueous sodium hydroxide solution and a 10% aqueous sodium chloride solution, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated under reduced pressure, and then the concen...
Embodiment 2
[0060] Embodiment 2 (the synthesis of 4-chloromethylenedioxybenzene)
[0061] 1.22 g (10 mmol) of methylenedioxybenzene and 1.22 g of acetic acid were added to a 25 ml glass flask equipped with a stirring device, a thermometer, and a reflux condenser under an argon atmosphere, and the temperature of the liquid was raised to 60°C. Then, keep the liquid temperature at 60-90°C, add 1.13g (5.5mmol) of 1,3-dichloro-5,5-dimethylhydantoin in small amounts each time, and react at 70°C for 1 hour under stirring . After the reaction, the reaction liquid was analyzed by high performance liquid chromatography (absolute quantitative method), and it was revealed that 1.34 g of 4-chloromethylenedioxybenzene was produced (reaction yield: 85.6%).
Embodiment 3
[0064] Embodiment 3 (the synthesis of 4-bromomethylenedioxybenzene)
[0065] 61.1 g (0.5 mol) of methylenedioxybenzene and 30 g of acetic acid were added to a 500-ml glass flask equipped with a stirring device, a thermometer, and a reflux condenser under an argon atmosphere, and the temperature of the liquid was raised to 60°C. Then, keep the liquid temperature at 60-90°C, add 1,3-dibromo-5,5-dimethylhydantoin 78.6g (0.275mol) in small amounts each time, and react at 70°C for 1 hour under stirring . After the reaction, the reaction solution was cooled to room temperature, and 200 g (1.0 mol) of 20% aqueous sodium hydroxide solution was added to the reaction solution, followed by stirring. Then, the reaction liquid was filtered, and the organic layer was separated, and then the organic layer was distilled under reduced pressure (93° C., 1200 Pa) to obtain 71.2 g of 4-bromomethylenedioxybenzene as a colorless liquid (isolation yield: 70.8%).
[0066] The physical property para...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com