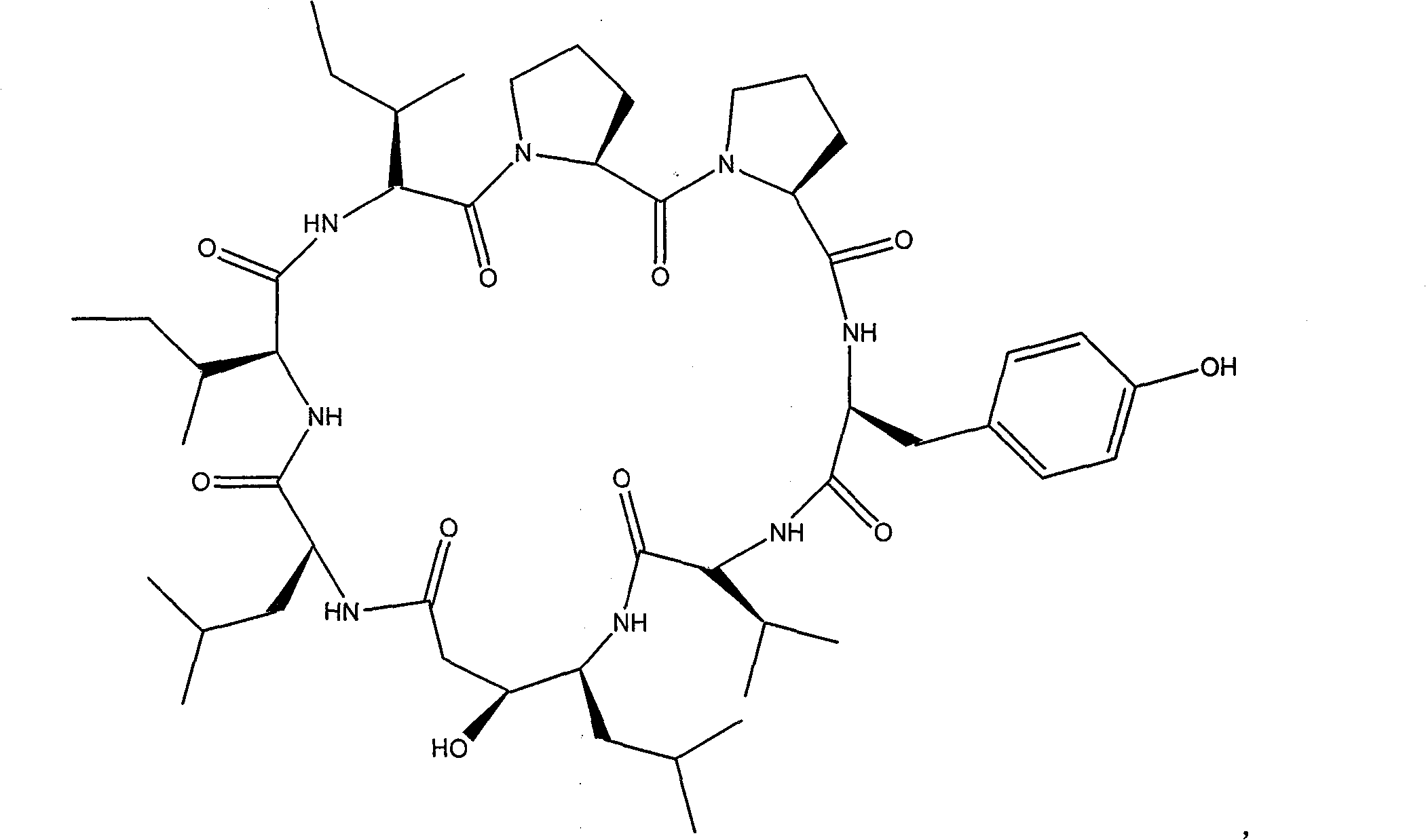
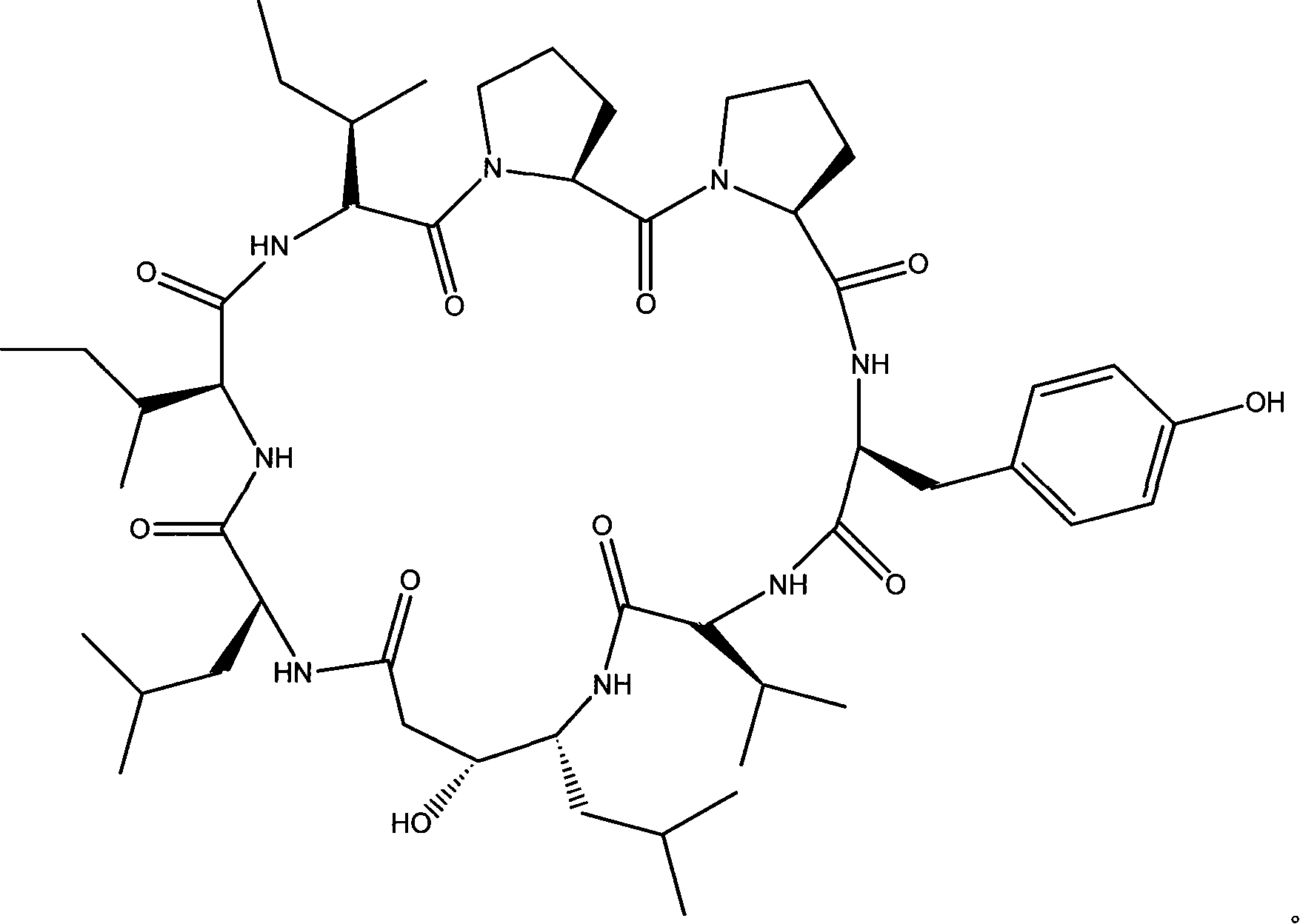
Cyclic peptide with -val-sta-leu- residue segment and used as immunity inhibitor and synthetic process thereof
An immunosuppressant, synthetic technology, applied in the fields of peptides, organic chemistry, drug combination, etc., can solve the problems of difficult chemical synthesis and unfavorable purification, and achieve the effect of stable physical and chemical properties and high purity
Active Publication Date: 2010-09-08
CHINESE PEPTIDE CO
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The amino acid residues in the natural structure of HS-1 are all hydrophobic amino acids, and the sequence is rich in Pro with a secondary amine ring structure, which makes chemical synthesis extremely difficult and is also very unfavorable for purification
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a cyclic peptide which is provided with a residue fragment of -Val-Sta-Leu- and is used as immunosuppressive agents, and the synthesis process of the cyclic peptide (Ile-Ile-Pro-Pro-Tyr-Val-Sta-Leu) consists of 1) removing amino-protecting groups, 2) transpeptidase reaction, 3) the cutting of a peptide chain, 4) cyclization of the peptide chain. The cyclic peptide compounds which are provided with the residue fragment of Val-Sta-Leu- and used as immunosuppressive agents and have a high yield and a high purity can be obtained, and the immunosuppressive activity of the cyclic peptide compounds is higher. Through the experiments of the retarding effect of A3HS1 towards the delayed hypersensitivity of mice, the influence of phagocytic function of macrophages, and the retarding effect of the proliferation of lymphocytes, the A3HS1 shows a stronger immunosuppressive effect than HS-1, which reaches or surpasses the immunosuppressive of the positive contrast (cyclosporine).
Description
technical field The invention relates to a peptide compound, specifically a cyclic peptide with a -Val-Sta-Leu- residue fragment that can be used as an immunosuppressant and a synthesis process. Background technique Hymenistatin-1 (HS-1) is a cyclic octapeptide isolated from the Pacific hymeniacidon sponge by researchers such as Pettit (Pettit G.R., Clewlow P.W., Dufresne C., et al. Isolation and structure of the cyclic peptide hymenistatin I . Can. J. Chem. 1990, 68: 708-711.). The sequence of the peptide chain is: cyclo(Pro-Pro-Tyr-Val-Pro-Leu-Ile-Ile). For the convenience and accuracy of discussion, the amino acid residues are numbered, that is, HS-1 is expressed as (Ile 1 -Ile 2 -Pro 3 -Pro 4 -Tyr 5 -Val 6 -Pro 7 -Leu 8 ). It has been reported that HS-1 has an inhibitory effect on cell growth in the study of P388 in mouse lymphoblastic non-leukemic leukemia, but the results have not been fully confirmed in subsequent studies. Researchers such as Siemion reve...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07K7/64C07K1/02C07K1/20A61P37/06
CPCY02P20/55
Inventor 李湘徐琪
Owner CHINESE PEPTIDE CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com