Compounds for inhibiting multi-medicine medicine-resistant staphylococcus aureus activity
A technology for multi-drug resistance and Staphylococcus aureus, applied in organic active ingredients, antibacterial drugs, organic chemistry, etc., can solve problems such as inability to play an antibacterial effect and lower drug concentration
Inactive Publication Date: 2010-11-10
FUDAN UNIV
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In these external pump mechanisms, bacterial cells "pump out" most types of antibiotic drug molecules commonly used, so that the concentration of the drug in the cell is reduced and the antibacterial effect cannot be exerted
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more PUM
 Login to view more
Login to view more Abstract
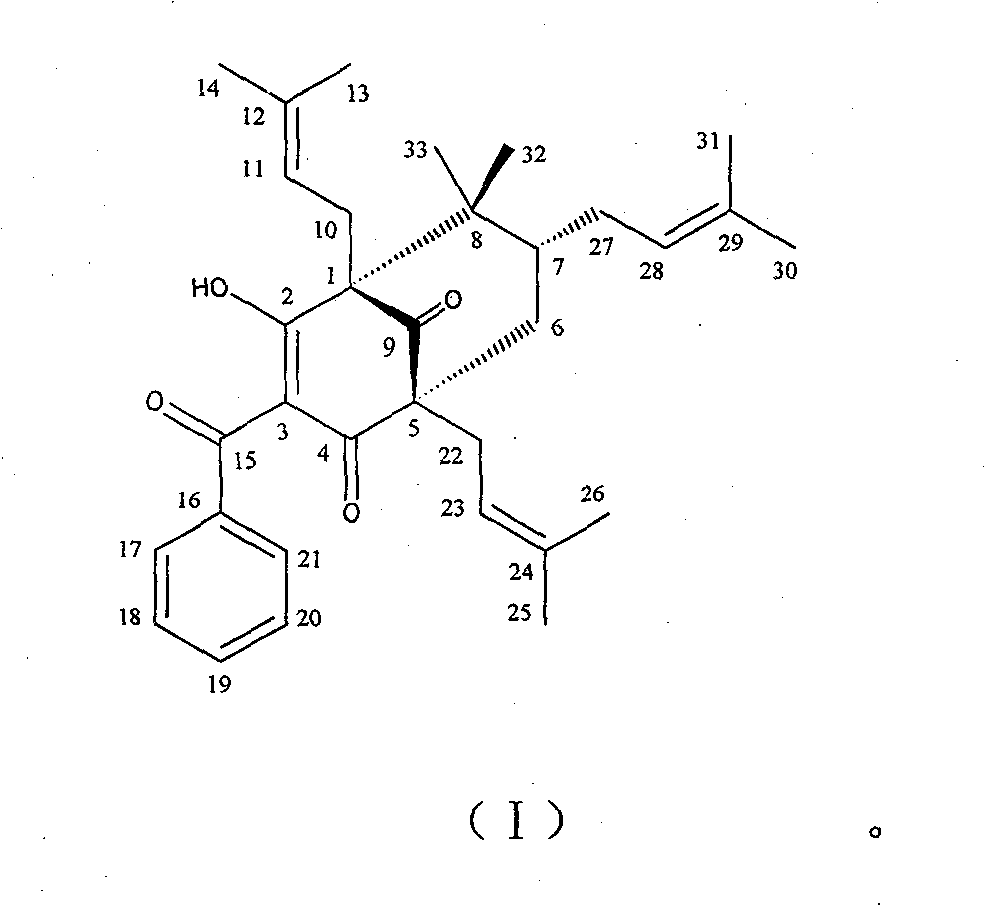
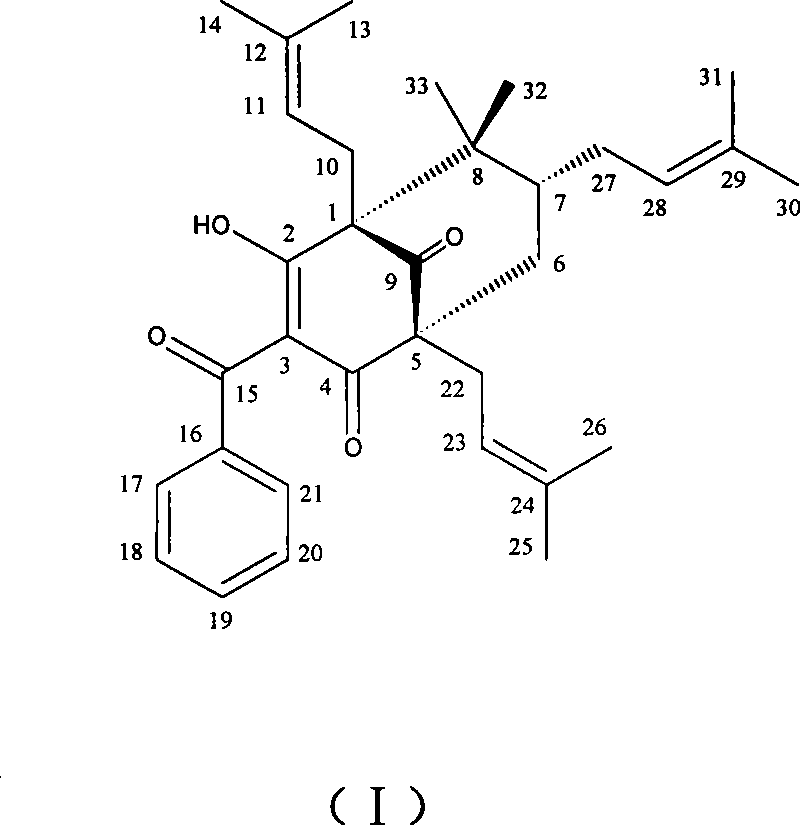
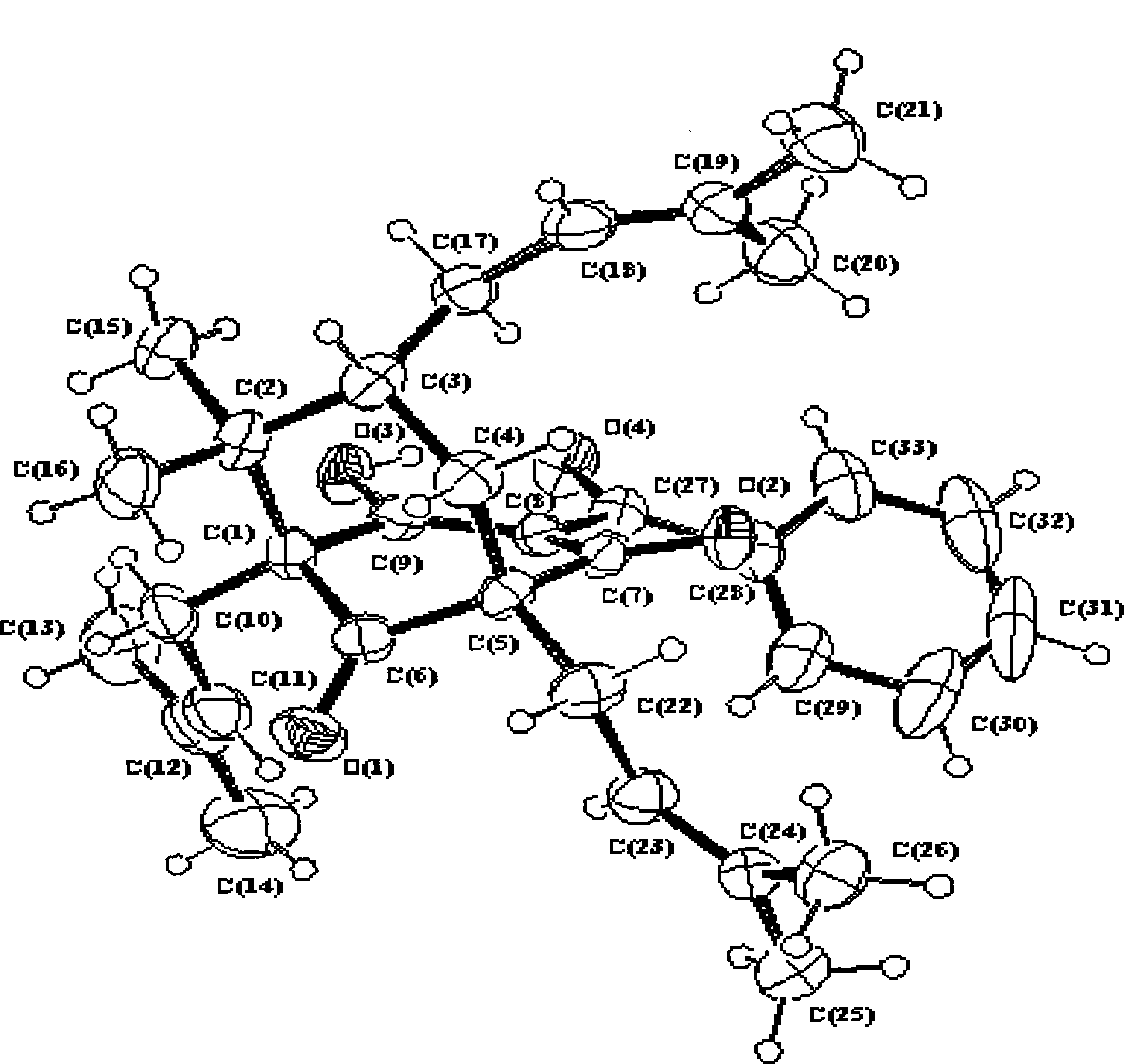
The invention belongs to the pharmaceutical field, and relates to compound of 1S<*>,2R<*>,7R<*>-3-benzoyl-2-hydroxy-8,8-dimethyl-1,5,7-tri(3-methyl-2-butenyl)-bbicyclo-[3.3.1]nonan-2-ene-4,9-dione. The compound has the molecular formula and the plane structure of 7-epiclusianone. The stereochemical configuration of a chiral carbon atom is the enantiomer of 7-epiclusianone, and is named as ent-7-epiclusianone. The pharmacological and bacteriostatic experimental results show that the compound has pharmacological activities for inhibiting multi-drug resistant Staphylococcus aureus; and for methi-cillin-resistant Staphylococcus aureus containing fluoroquinolone-resistant genes NorA, the minimum inhibitory concentration (MIC) of the compound is only of 4 Mu g / mL (7.3 Mu M) and the compound activity is higher than a contrast drug when the MIC of norfloxacin positive contrast drug is of 32 Mu g / mL (100 Mu M). The compound can be used for preparing antibacterial injections and external pharmaceutical preparations.
Description
A compound that inhibits the activity of multidrug-resistant staphylococcus aureus technical field The invention belongs to the field of pharmacy and relates to compound 1S*, 2R*, 7R*-3-benzoyl-2-hydroxyl-8,8-dimethyl-1,5,7-tri(3-methyl-2- Butenyl)-bicyclo[3.3.1]non-2-ene-4,9-dione, named after ent-7-epiclusianone (hereinafter referred to as HSRO1-1), and its use in the preparation of anti-fluoronoquinones Use in medicines for methicillin-resistant Staphylococcus aureus (MRSA). Background technique Antimicrobial resistance is a growing problem. Methicillin-resistant Staphylococcus aureus (MRSA) has become a common and serious infectious bacteria in hospitals in recent years. In addition to superficial skin infections, it also has deep infections inside the human body, which can lead to death in severe cases. Due to the widespread use and even abuse of antibiotics in my country in the past one or two years, especially after vancomycin has become a routine antibiotic drug...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to view more Application Information
Patent Timeline
 Login to view more
Login to view more Patent Type & Authority Patents(China)
IPC IPC(8): C07C49/83A61P31/04A61K31/122
Inventor 穆青肖志勇曾祎含
Owner FUDAN UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Try Eureka
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap