Anti-tumor and antibacterial compound and application thereof
A compound and anti-tumor technology, applied in the direction of anti-tumor drugs, anti-bacterial drugs, hybrid peptides, etc., can solve problems such as differences and achieve strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
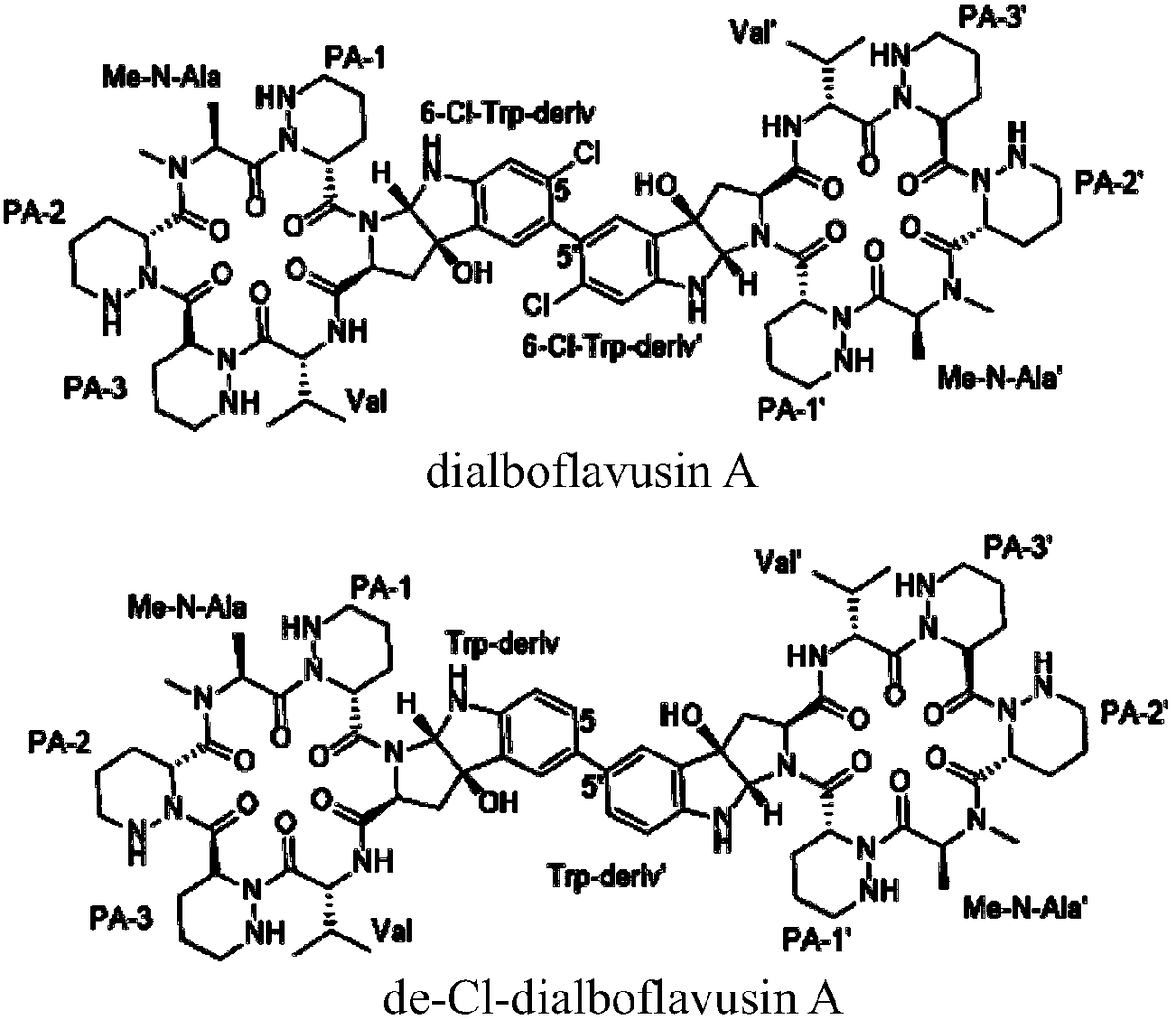
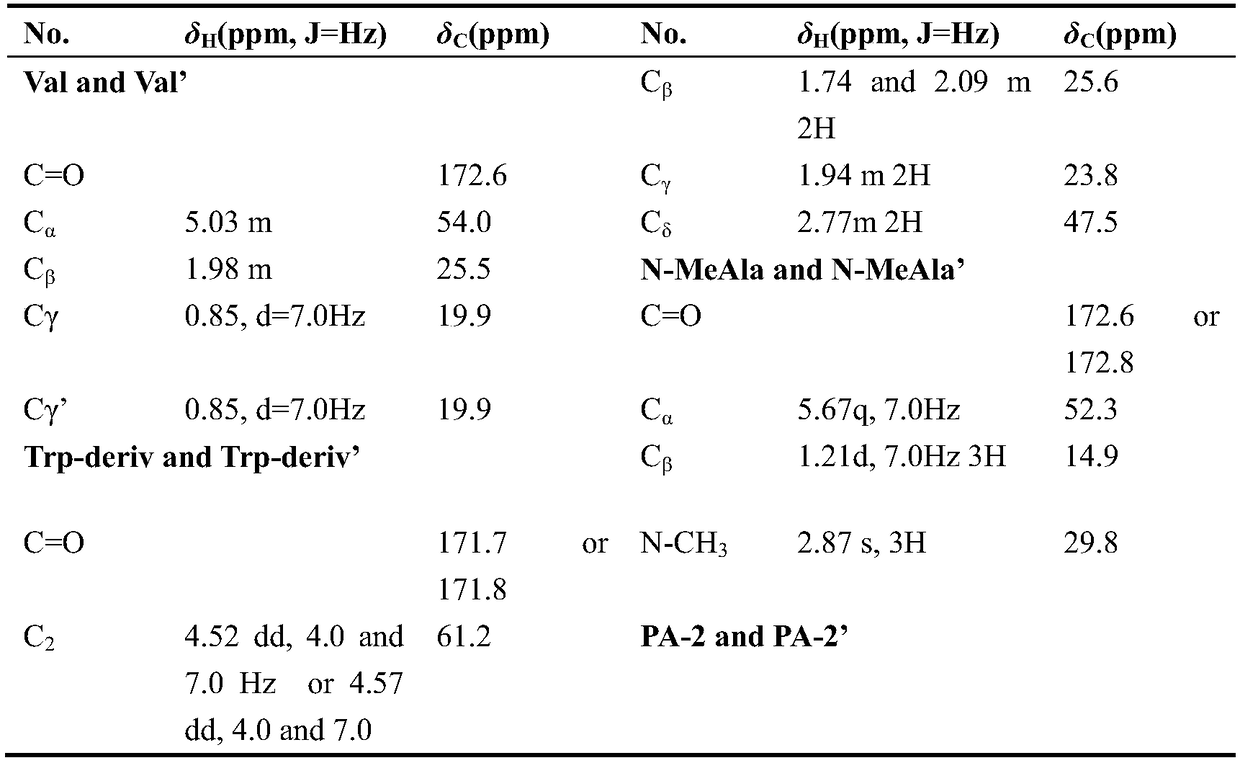
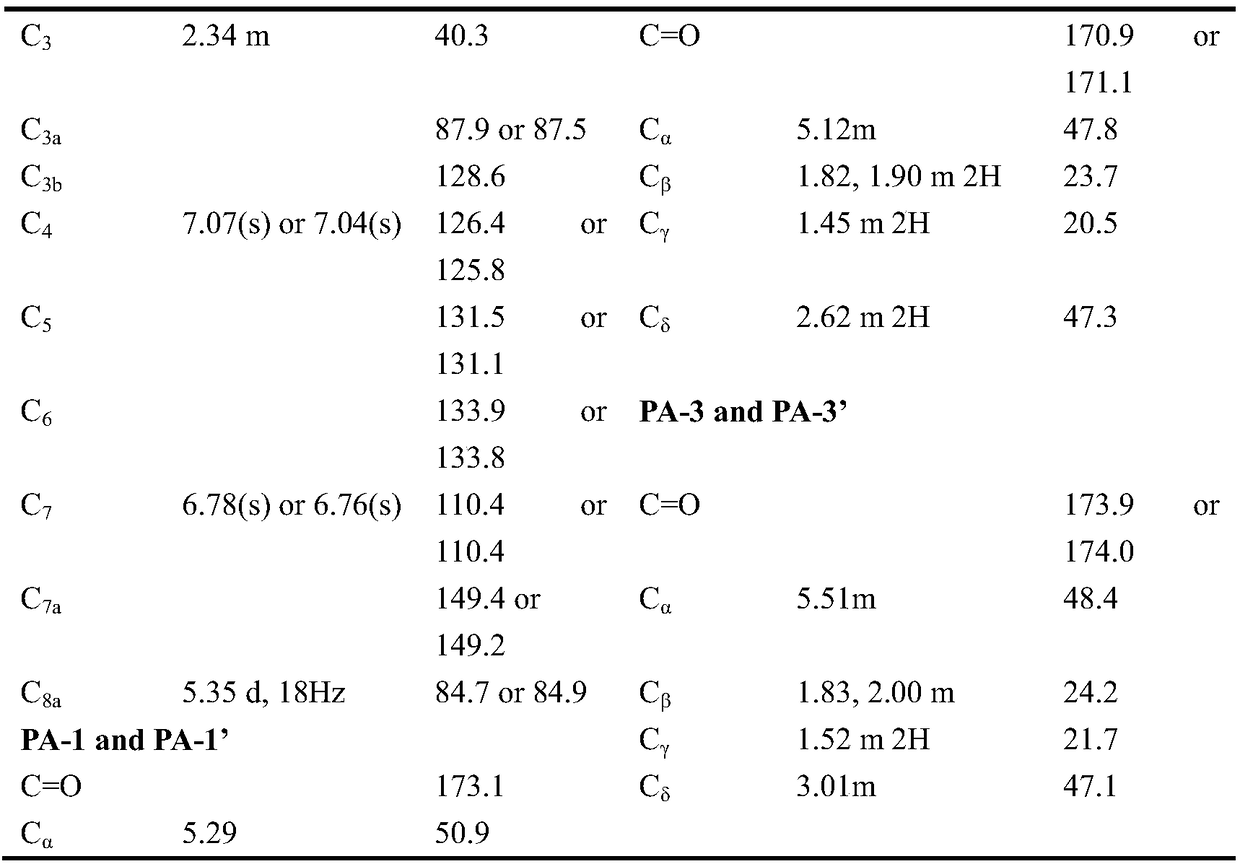
[0022] Example 1 : Isolation and structure identification of Dialboflavusin A and de-Cl-dialboflavusin A
[0023] 1.1 Isolation and structure identification of Dialboflavusin A
[0024] Streptomyces alboflavus 313-HmtS mutant strain fermentation broth (60L) was centrifuged to remove bacteria (5000 rpm, 30 minutes), and 2kg HP20 macroporous resin was used to absorb and enrich the active ingredients, first eluted with 5L distilled water, and the eluate was discarded . Then 10 L was eluted with methanol. Concentrate 10L of the methanol eluate to dryness, perform silica gel column chromatography, and use ethyl acetate, ethyl acetate / methanol (50% / 50%, volume ratio) and methanol system for elution, and 5 L of each fraction is eluted. Concentrate ethyl acetate / methanol (50% / 50%, volume ratio) to a small volume, filter through a 0.45-micron filter membrane, and perform dextran gel SephexLH20, eluting with methanol at a flow rate of 1 mL / min, and each fraction is 5 mL. The fractio...
Embodiment 2
[0037] Example 2 : Antibacterial activity of Dialboflavusin A and de-Cl-dialboflavusin A
[0038] The effect of dialboflavusin A and de-Cl-dialboflavusin A on Bacillus subtilis (Bacillus subtilis BS168), Bacillus cereus (CGMCC 1.0230), Staphylococcus aureus (Staphylococcus aureus ATCC 6538) and four different resistant Methicillin-resistant Staphylococcus aureus methicillin-resistant Staphylococcus aureus 113 (MRSA-1), methicillin-resistant Staphylococcus aureus 1.2386 (MRSA-2), methicillin-resistant Staphylococcus aureus 09R496 (MRSA-3), and methicillin-resistant Staphylococcus aureus (08Lucoccus aureus MRSA-4) and other seven kinds of bacteriostatic activity of tested Gram-positive bacteria. Methicillin-resistant Staphylococcus aureus was provided by the State Key Laboratory of Early Development of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences, and the rest of the strains were purchased from the China General Microbiology Collection Center.
...
Embodiment 3
[0044] Example 3 : Dialboflavusin A and de-Cl-dialboflavusin A inhibit the activity of tumor cells.
[0045] MTT method was used to determine the effects of dialboflavusin A and de-Cl-dialboflavusin A on cervical cancer cell line Hela, human ovarian adenocarcinoma cell line SKOV3, human breast cancer cell line MCF-7, human lung cancer cell line A549, human liver cancer cell line Antitumor activity of HepG2 line, human glioma cell line U251 and human gastric cancer cell line SGC-7901. All tumor cell lines were obtained from the Institute of Basic Medical Sciences, Chinese Academy of Military Sciences.
[0046] Tumor cell lines were formulated to 5×10 3 100 μL of each concentration of the suspension was inoculated into each well of a 96-well plate, cultured overnight, and the samples of dialboflavusin A and de-Cl-dialboflavusin A at the concentration to be tested were added, and 5-fluorouracil was used as a positive control. After culturing for 72 hours, Add 20 μL of MTT rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com