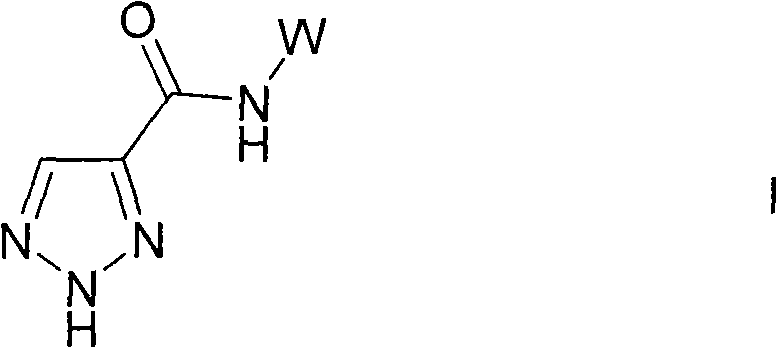Triazole compounds as lipoxygenase inhibitors
A compound, optionally a technology, applied in the direction of anti-inflammatory agents, drug combinations, organic chemistry, etc., can solve problems that are not specifically disclosed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 69
[0441] general steps
[0442] Method A
[0443]TBTU (1.1 mmol) was added to a solution of 1,2,3-triazole-4-carboxylic acid (113 mg, 1.0 mmol) and diisopropylethylamine (258 mg, 2 mmol) in anhydrous DMF (1 mL) and The mixture was stirred at room temperature for 10 min. The relevant arylamine (1.3 mmol) was added and the mixture was stirred at the indicated temperature for the indicated period. The resulting mixture was concentrated and water (20 mL) was added to the residue. The mixture was extracted with EtOAc (3 x 20 mL) and the combined extracts were washed with water (20 mL), dried (Na 2 SO 4 ) and concentrate. The residue was purified by chromatography (eluent EtOAc / heptane) to afford the title product.
[0444] Method B
[0445] 1,2,3-triazole-4-carboxylic acid (65 mg, 0.50 mmol), SOCl 2 (1 mL) and DMF (1 drop) was heated at 40 °C for 2 h. The mixture was concentrated and the residue was dried in vacuo. The resulting solid, DMAP (83mg, 0.68mmol) and the re...
Embodiment 70-78
[0469] general steps
[0470] (a) 3-[2-(Trimethylsilyl)ethoxymethyl]-1,2,3-triazole-4-carboxylic acid aryl-amide
[0471] Butyllithium (1.6M in hexane, 1.1 mL, 1.7 mmol) was added dropwise to 1-[2-(trimethylsilyl)ethoxymethyl]-1 cooled to -20 °C, 2,3-Triazole (3:1 mixture of isomers prepared as described above, 300 mg, 1.5 mmol) in THF (20 mL). The mixture was stirred at -20°C for 30 min and cooled to -78°C. A solution of the relevant aryl isocyanate (2.0 mmol) in THF (5 mL) was added dropwise and the mixture was stirred at -78 °C for 2 h, allowed to warm to room temperature, and then stirred at room temperature for 18 h. Add Et 2 O (20mL) and NH 4 Cl (aq, sat, 10 mL) and the layers were separated. Wash the aqueous phase with Et 2 O (2×20 mL) was extracted and the combined extracts were dried (Na 2 SO 4 ) and concentrate. The residue was purified by chromatography (eluent EtOAc / heptane) to afford the subtitled product (intermediate (a) 32 to 40) as a white or yell...
Embodiment 79-105
[0483] general steps
[0484] Butyllithium (1.6M in hexane, 900 μL, 1.5 mmol) in THF (12 mL) cooled to -50 °C was added dropwise to 1-[2-(trimethylsilyl)ethoxy A solution of methyl]-1,2,3-triazole (3:1 mixture of isomers, prepared as described above, 210 μL, 299 mg, 1.5 mmol). The mixture was stirred at -50°C for 30 min, cooled to -78°C and a solution of the relevant isocyanate (2 mmol) in THF (5 mL) was added dropwise. The mixture was stirred at -78 °C for 30 min, allowed to warm to room temperature and stirred at room temperature for 16 h. The mixture was cooled to 0 °C and HCl (10 mL of 0.27M in EtOH, 2.7 mmol) was added. After stirring at 0 °C for 4 h, the mixture was concentrated and the residue was purified by chromatography (eluent EtOAc / heptane, 20-60%) to afford the title product.
[0485] Table 5 - Examples (Ex.) 79 to 105
[0486] Ex.
[0487]
[0488]
[0489] Table 6 - Physical Properties of Examples 79-105
[0490] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com