Vaccines comprising truncated HBC core protein plus saponin-based adjuvants
A saponin and adjuvant technology, applied in the field of vaccines containing truncated HBC core protein and saponin-based adjuvants, can solve problems such as weak immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
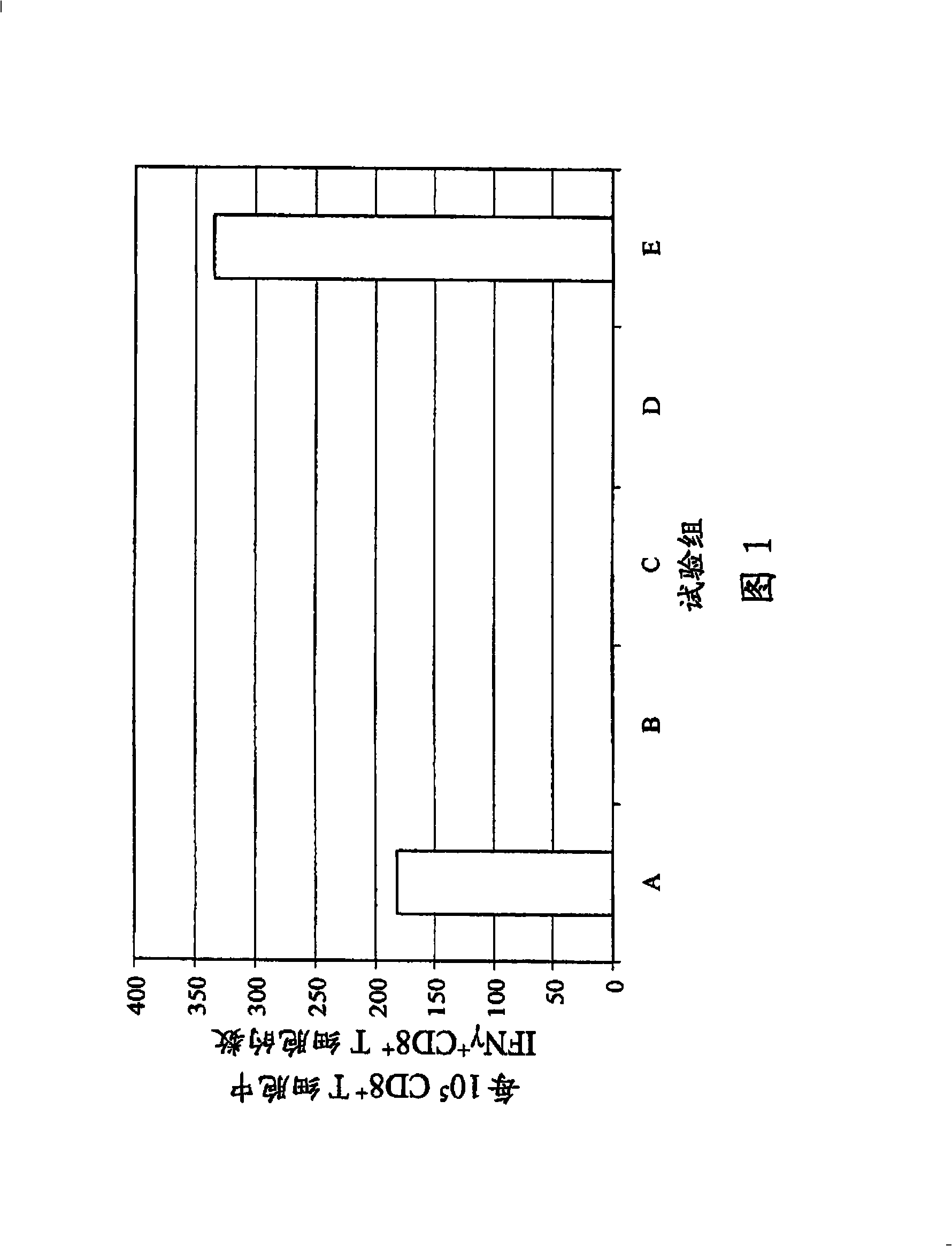
[0148] In the first series of experiments, in the product description AbISCO A commercially available saponin complex at -100 (ISCONOVA, Uppsala, Sweden) was used as adjuvant. Core plasmid DNA was used as a positive control, pure PBS, pure adjuvant in PBS, pure HBc in PBS 1-144+I And HBcl-183 in PBS served as a negative control. The composition of the present invention comprises PBS, HBc 1-144+I and AbISCO -100. The amount of each component is shown in Table 1.
[0149] Plasmid pCI / HBV ayw Core basis A., Pudollek H.P., Reifenberg K., Chisari F.V., Schlicht H.J., Reimann J., Schirmbeck R. (1996): DNA Immunization Induces Antibodies and Cytotoxic T Cells to Hepatitis B Core Antigen in H-2b Mice Reaction", J.Immunol.156:3687-95 (the construct is called pCMV-1 / c there), its isolation was carried out according to Carried out according to the manufacturer's instructions.
[0150] For the formulation of the composition of the present invention, HBc 1-144+I First dilute t...
Embodiment 2
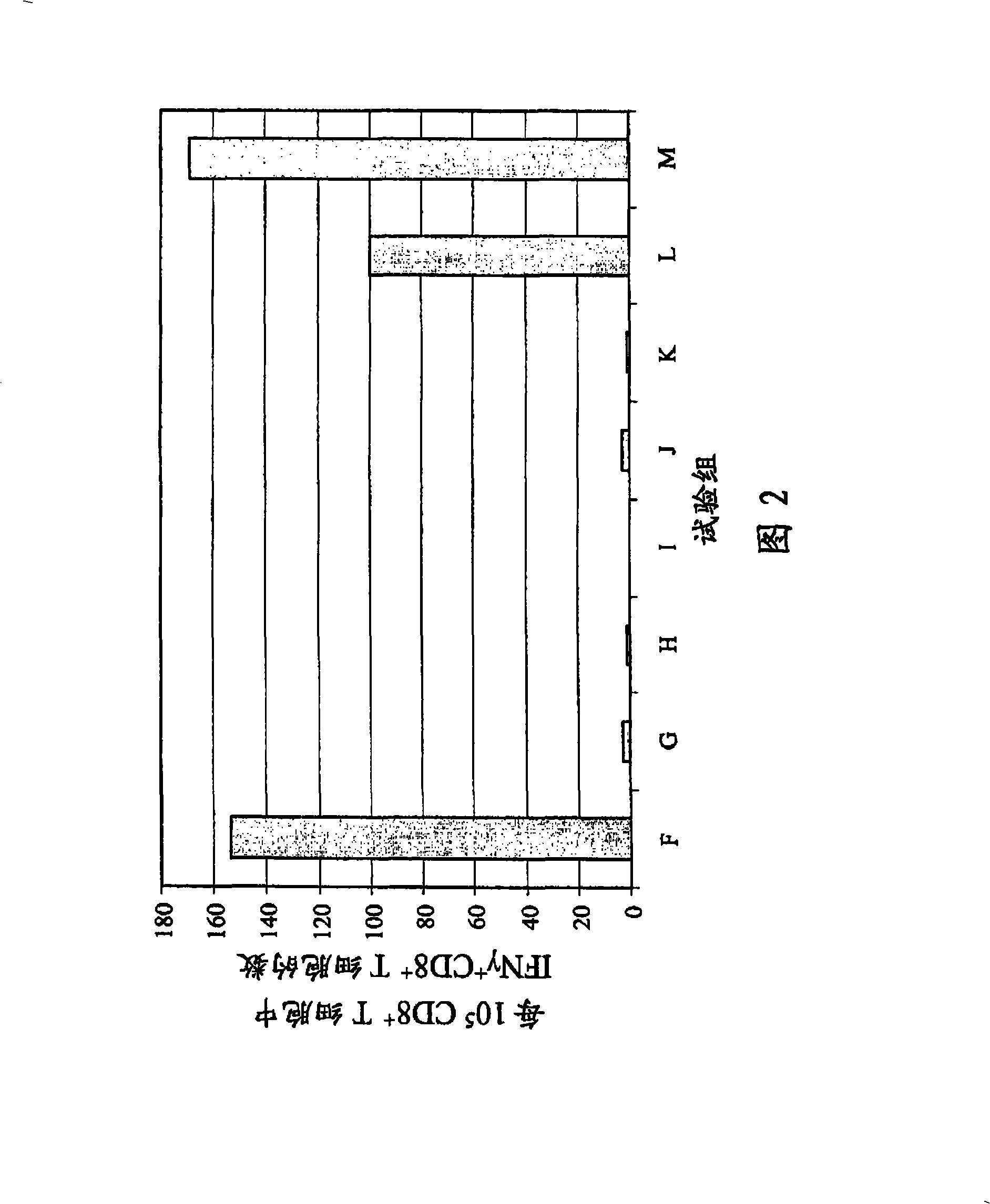
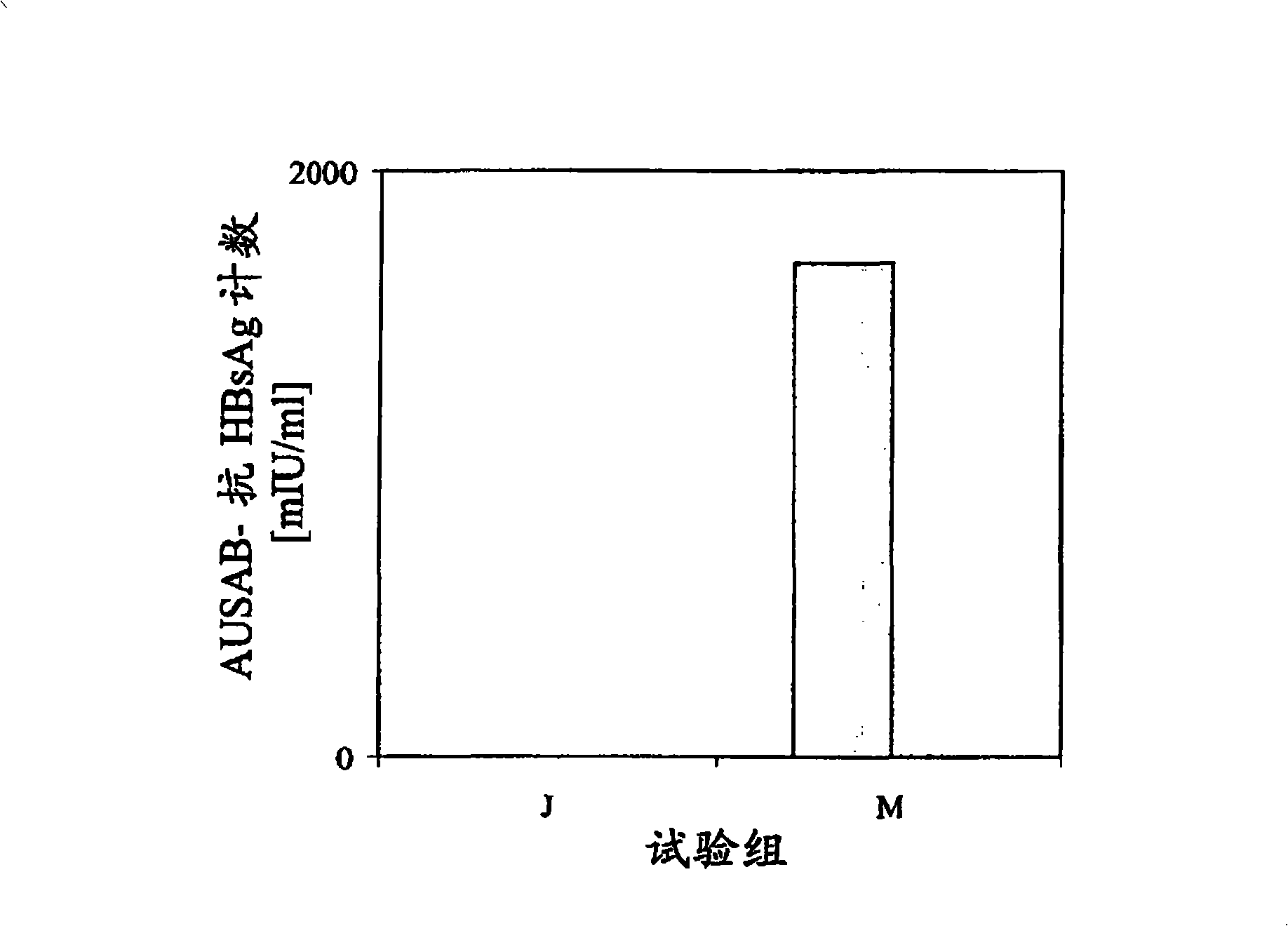
[0156] In the following series of experiments, in the product description AbISCO A commercially available saponin complex at -200 (ISCONOVA, Uppsala, Sweden) was used as adjuvant. Core plasmid DNA as positive control; pure PBS, pure adjuvant in PBS, pure HBC in PBS 1-144+I and pure HBc in PBS 1-183 as a negative control. The composition of the present invention comprises PBS, HBc 1-144+I , AbISCO -200 and optionally HBsAg. The amount of each individual component is shown in Table 2.
[0157] For the formulation of the composition of the present invention, HBc 1-144+I And optionally the HBsAg is firstly diluted with PBS to 4 times the final product concentration, and incubated at 37° C. for 30 minutes with shaking. When using HBsAg, first, HBsAg and HBcAg 1-144+I Mix under sterile conditions and dilute likewise with PBS to 4 times the final product concentration. AbISCO -200 (in the form of an aqueous dispersion supplied by the manufacturer) was then added and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com