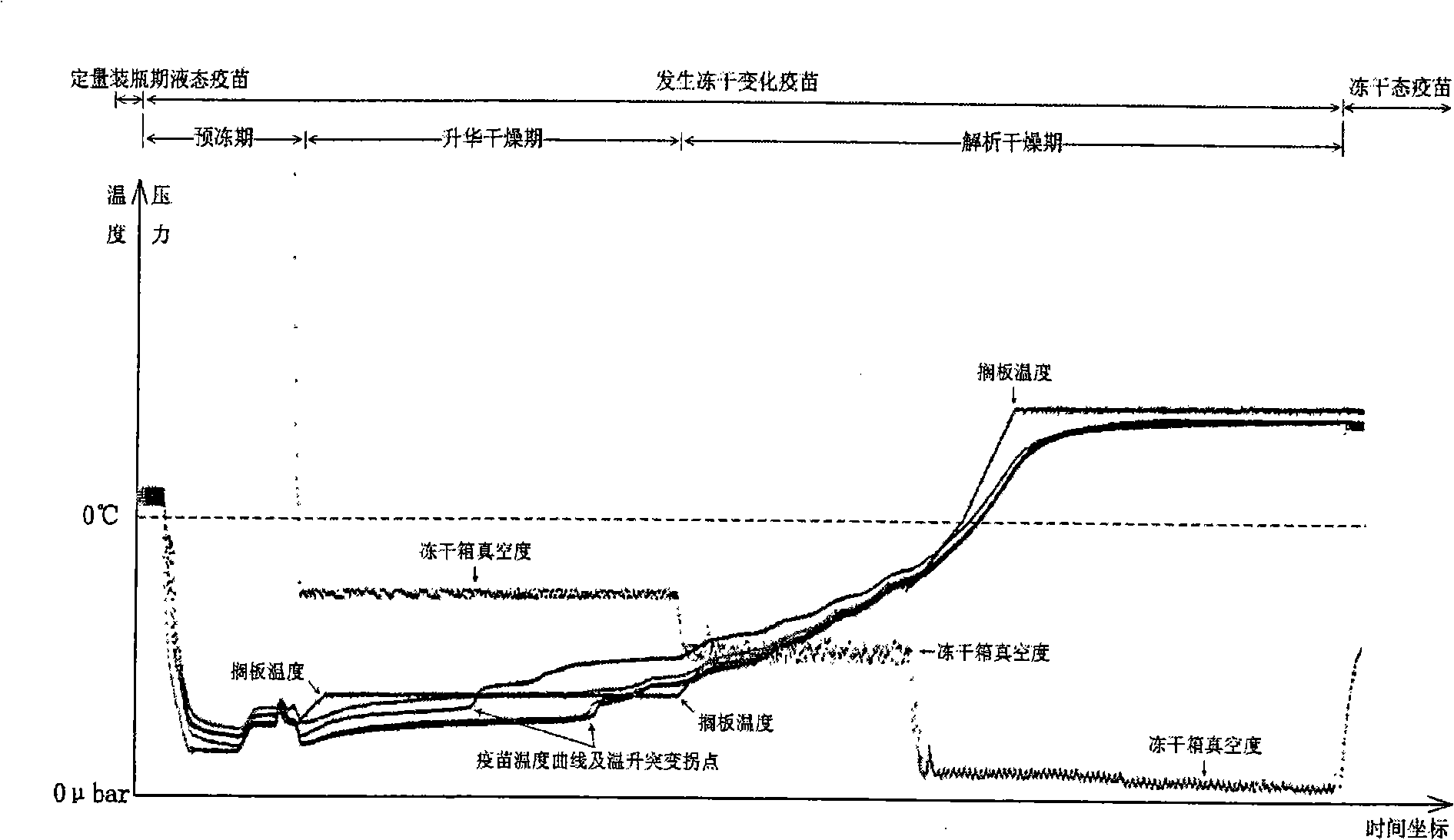
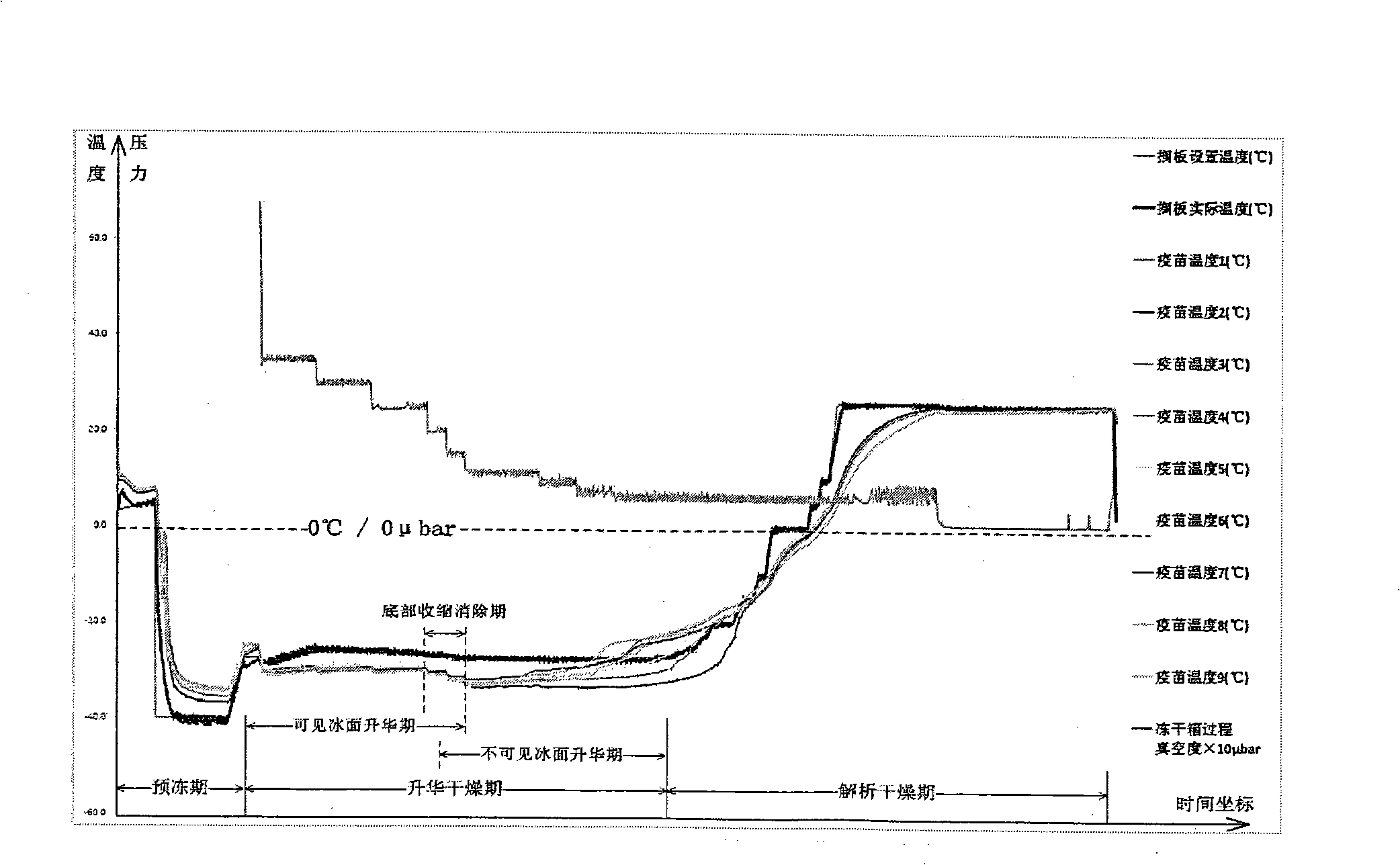
Lyophilization technique for preparing lyophilized hepatitis A attenuated live vaccine
A technology of live attenuated vaccines and hepatitis A, which is applied in freeze-dried delivery, antiviral agents, and resistance to vector-borne diseases, etc. It can solve the problems of vaccine infectious titer loss, shrinkage, and production cost impact, and achieve elimination The problem of large loss of infectious titer, reduction of loss of infectious titer, and the effect of economical and reasonable production capacity upgrade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The present invention adopts the following steps to carry out freeze-drying to the semi-finished liquid hepatitis A live attenuated vaccine (commercially available product) of packing, packing capacity 54358 bottles, freeze-drying cycle time 26 hours and 40 minutes:
[0035] (1). Control the shelf temperature of the freeze-drying box of the freeze-dryer to 5°C, and load the half-finished and half-stoppered liquid hepatitis A live attenuated vaccine layer by layer until all the shelves are fully loaded, and then turn off the freeze-drying door, the freeze-drying process starts;
[0036] (2). Control the shelf temperature of the freeze-drying box to 5°C and keep it for 60 minutes;
[0037] (3). Lower the shelf temperature of the freeze-drying box to -40°C at a rate of 1.5°C per minute, and keep the temperature at -40°C for 90 minutes when the shelf temperature reaches -40°C;
[0038] (4). Raise the shelf temperature from -40°C to -24.9°C at a rate of about 0.5°C per minu...
Embodiment 2
[0074] Adopt the following steps to freeze-dry the semi-finished liquid hepatitis A live attenuated vaccine (commercially available product) after subpackaging, packing capacity 55000 bottles, freeze-drying cycle time 21.5~22.5 hours:
[0075] (1). Control the temperature of the freeze-drying box of the freeze-dryer at 10°C, divide the bottles filled with semi-finished and half-stoppered liquid hepatitis A live attenuated vaccine layer by layer until all the shelves are fully loaded, and then turn off the freeze-drying door, the freeze-drying process starts;
[0076] (2). Control the shelf temperature of the freeze-drying box to 10°C and keep it for 30 minutes;
[0077] (3). Lower the shelf temperature of the freeze-drying box to -40°C at a rate of 1.5°C per minute, and keep the shelf temperature at -40°C for 90 minutes;
[0078] (4). The shelf temperature is raised from -40°C to -24.9°C at a rate of 0.60°C per minute, and the heating time is 30 minutes;
[0079] (5). Then l...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap