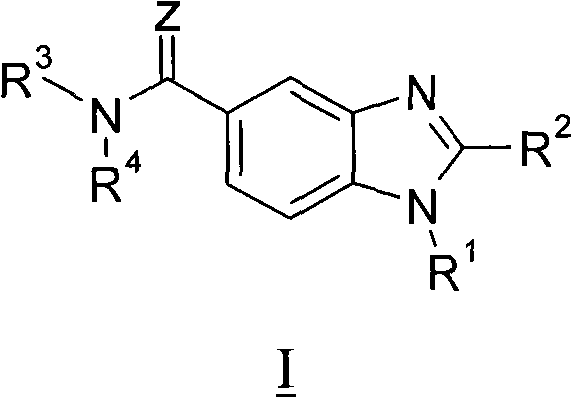
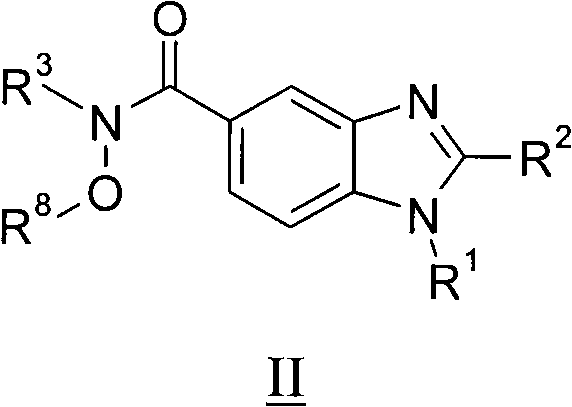
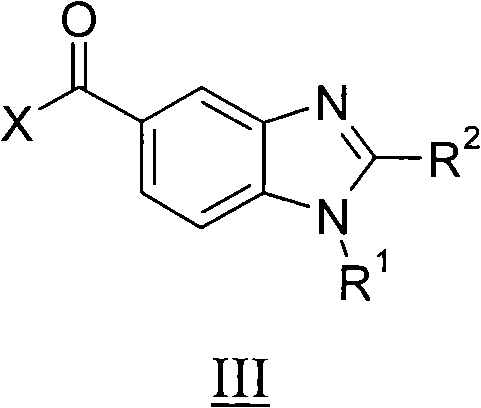
Benzimidazole derivatives, compositions containing them, preparation thereof and uses thereof
A compound, phenyl technology, applied in the field of therapeutic compounds, which can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] 2-tert-butyl-N, N-diethyl-1-{[(2R)-1-methylpiperidin-2-yl]methyl}-1H-benzimidazole-5-carboxamide
[0179]
[0180] Step A. 2-tert-butyl-N,N-diethyl-1-{[(2R)-1-methylpiperidin-2-yl]methyl}-1H-benzimidazole-5-carboxamide
[0181]
[0182] (2R)-tert-butyl 2-[({2-amino-4-[(diethylamino)carbonyl]phenyl}amino)methyl]piperidine-1-carboxylate (75 mg, 0.185 mmol) (for Preparation, see steps B, C and D below) Dissolved in 3 mL of DCE containing TEA (0.058 mL, 0.277 mmol). Trimethylacetyl chloride (0.025 mL, 0.204 mmol) was added dropwise, and the solution was stirred at room temperature for 1 hour. Glacial acetic acid (1 mL) and a few drops of concentrated HCl were added, and the solution was stirred at 75°C for 24 hours. The solvent was concentrated. The residue was dissolved in EtOAc and washed with 2M NaOH, brine, and washed with anhydrous MgSO 4 dry. The solvent was evaporated. The product was dissolved in 5 mL of THF containing a few drops of glacial AcOH. Excess ...
Embodiment 2
[0193] 2-tert-butyl-1-(cyclohexylmethyl)-N,N-diethyl-1H-benzimidazole-5-carboxamide
[0194]
[0195] Step A. 2-tert-butyl-1-(cyclohexylmethyl)-N,N-diethyl-1H-benzimidazole-5-carboxamide
[0196]
[0197] 3-Amino-4-[(cyclohexylmethyl)amino]-N,N-diethylbenzamide (124 mg, 0.409 mmol) (for preparation, see steps B and C below) was dissolved in 3 mL of TEA containing (0.085 mL, 0.614 mmol) in DCE. Trimethylacetyl chloride (0.055 mL, 0.450 mmol) was added dropwise, and the solution was stirred at room temperature for 1 hour. Glacial AcOH (1 mL) and a few drops of concentrated HCl were added, and the solution was stirred at 75°C for 48 hours. The solvent was concentrated. The residue was dissolved in EtOAc and washed with 2M aqueous NaOH, brine, and washed with anhydrous MgSO 4 dry. The solvent was evaporated. The product was obtained by using 20-80% CH 3 CN / H 2 Reverse phase HPLC purification of O and then lyophilization afforded the desired title compound as the corr...
Embodiment 3
[0205] 2-tert-butyl-1-(cyclohexylmethyl)-N-methoxy-N-methyl-1H-benzimidazole-5-carboxamide
[0206]
[0207] Step A. 2-tert-Butyl-1-(cyclohexylmethyl)-N-methoxy-N-methyl-1H-benzimidazole-5-carboxamide
[0208]
[0209] 2-tert-Butyl-1-(cyclohexylmethyl)-1H-benzimidazole-5-carboxylic acid (58 mg, 0.184 mmol) (for preparation see steps B-F below), N,O-dimethylhydroxy Amine hydrochloride (25 mg, 0.276 mmol) and HATU (77 mg, 0.202 mmol) were stirred in 2 mL of DIPEA (0.080 mL, 0.460 mmol) in DMF at room temperature for 3 hours. The solvent was concentrated. The residue was dissolved in EtOAc and washed with saturated NaHCO 3 solution, brine, and anhydrous MgSO 4 dry. The solvent was evaporated. Pass the product on a C-18 column by using 20-80% CH 3 CN / H 2 Reverse phase HPLC purification of O and then lyophilization afforded the desired title compound as the corresponding TFA salt. Yield: 29 mg (33%). 1 H NMR (400MHz, methanol-D 4 )δ1.23(m, 5H), 1.62(s, 2H), 1.65(s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com