Process for producing high-purity diphenyl carbonate on an industrial scale
一种碳酸二苯酯、工业规模的技术,应用在高纯度碳酸二苯酯的工业制备领域,能够解决收率降低、聚碳酸酯原料不合适、不是工业规模等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0384] (1) Process for continuous preparation of dimethyl carbonate and ethylene glycol (I)
[0385] 0 >
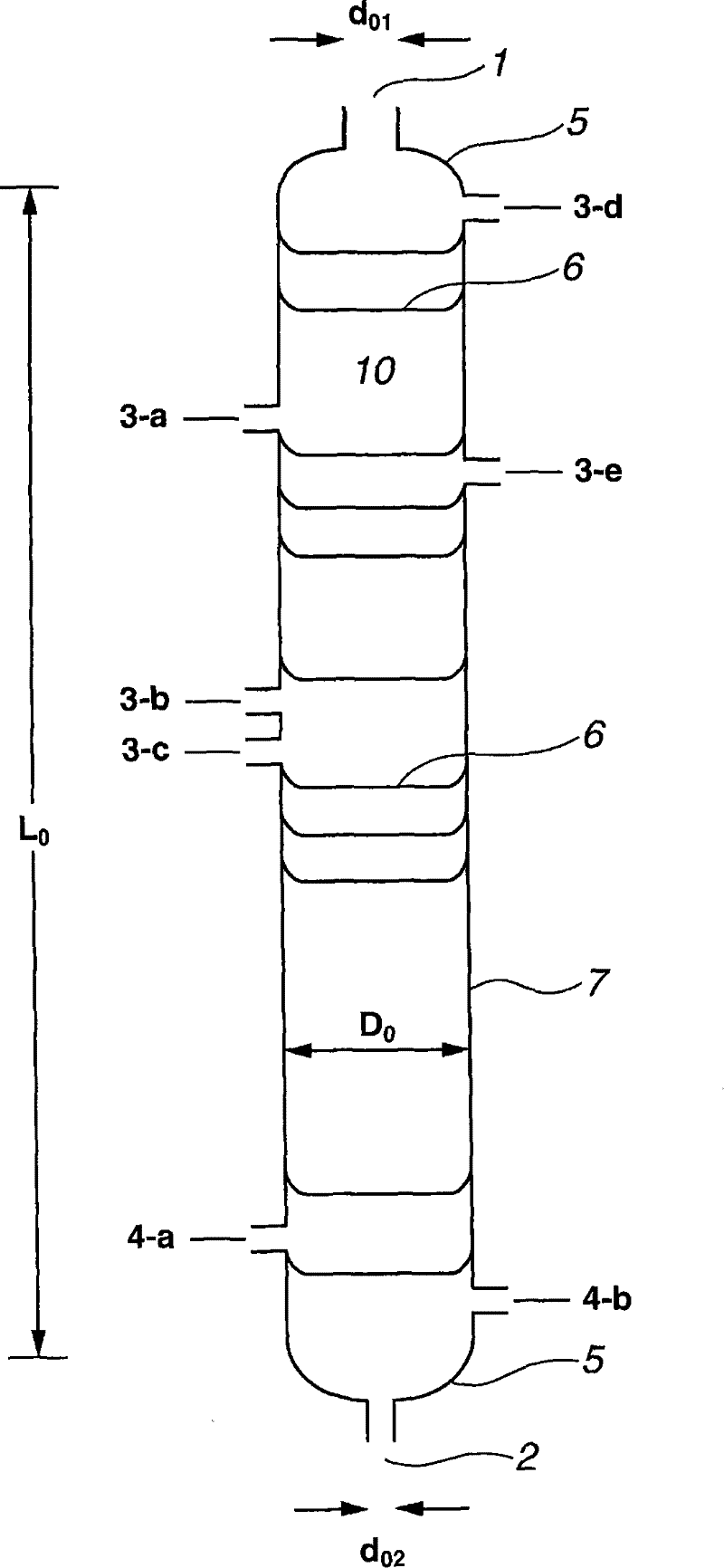
[0386] use figure 1 L as shown 0 = 3300cm, D 0 =300cm, L 0 / D 0 =11, n 0 = 60, D 0 / d 01 =7.5, D 0 / d 02 = 12 continuous multi-stage distillation column. In addition, in this embodiment, a perforated tray is used as an internal part, and the cross-sectional area of each hole of the perforated plate is approximately = 1.3 cm 2 , The number of holes is approximately = 180~320 / m 2 .
[0387]
[0388] From the inlet (3-a) at the 55th stage from the bottom to the distillation tower T 0 Continuously introduce 3.27 tons / hour of liquid ethylene carbonate. From the inlets (3-b and 3-c) at the 31st stage from the bottom to the distillation tower T 0 3.238 tons / hour of gaseous methanol (containing 8.96 mass% of dimethyl carbonate) and 7.489 tons / hour of liquid methanol (containing 6.66 mass% of dimethyl carbonate) were continuously introduced. Into the distillation tower T 0 The molar ...
Embodiment 2
[0416] (1) Process for continuous preparation of dimethyl carbonate and ethylene glycol (I)
[0417] Using the same continuous multi-stage distillation column as in Example 1, reactive distillation was performed under the following conditions.
[0418] 2.61 tons / hour of liquid ethylene carbonate was continuously introduced into the distillation column from the inlet (3-a) provided at the 55th stage from the bottom. Continuously introduce 4.233 tons / hour of gaseous methanol (containing 2.41% by mass of dimethyl carbonate) and 4.227 tons / hour of liquid methanol into the distillation tower from the inlets (3-b and 3-c) located at the 31st stage from the bottom. (Contains 1.46% by mass of dimethyl carbonate). The molar ratio of the raw materials introduced into the distillation column is: methanol / ethylene carbonate=8.73. As in Example 1, the catalyst was continuously supplied to the distillation column. The temperature at the bottom of the tower is 93℃, and the pressure at the top ...
Embodiment 3
[0429] (1) Use figure 1 L as shown 0 = 3300cm, D 0 =300cm, L 0 / D 0 =11, n 0 = 60, D 0 / d 01 =7.5, D 0 / d 02 = 12 continuous multi-stage distillation column. In addition, in this embodiment, a perforated tray is used as an internal part, and the cross-sectional area of each hole of the perforated plate is approximately = 1.3 cm 2 , The number of holes is approximately = 220~340 / m 2 .
[0430] 3.773 tons / hour of liquid ethylene carbonate was continuously introduced into the distillation column from the inlet (3-a) provided at the 55th stage from the bottom. Continuously introduce 3.736 tons / hour of gaseous methanol (containing 8.97% by mass of dimethyl carbonate) and 8.641 tons / hour of liquid methanol into the distillation tower from the inlets (3-b and 3-c) at the 31st stage from the bottom. (Contains 6.65% by mass of dimethyl carbonate). The molar ratio of the raw materials introduced into the distillation column is: methanol / ethylene carbonate=8.73. The catalyst was the sam...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com