Amphipathic squarylium cyanine dyes, its preparation method and use thereof
A technology of squaraine and dyes, applied in squaraine dyes, azo dyes, organic dyes, etc., which can solve the problems of low fluorescence quantum yield and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
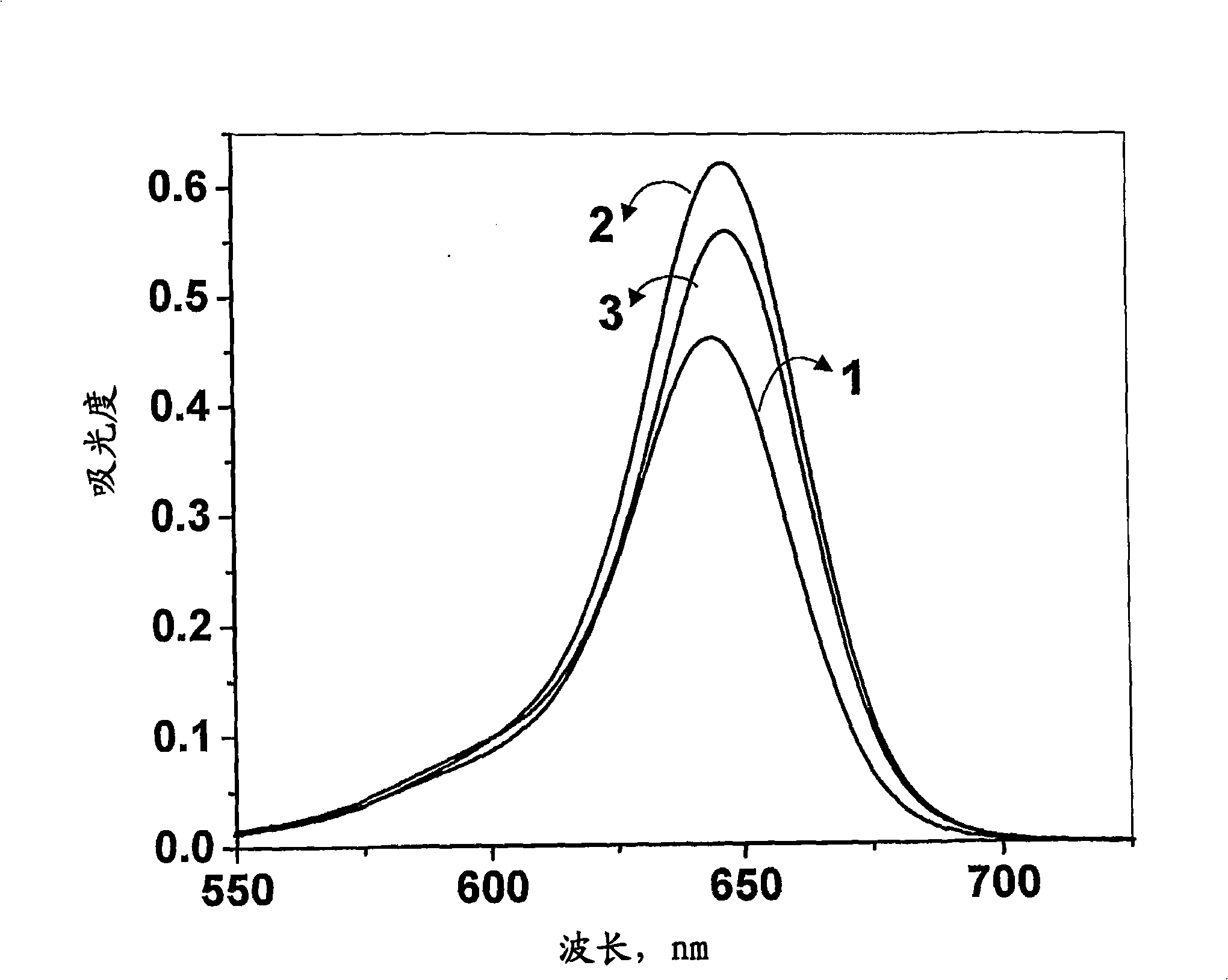
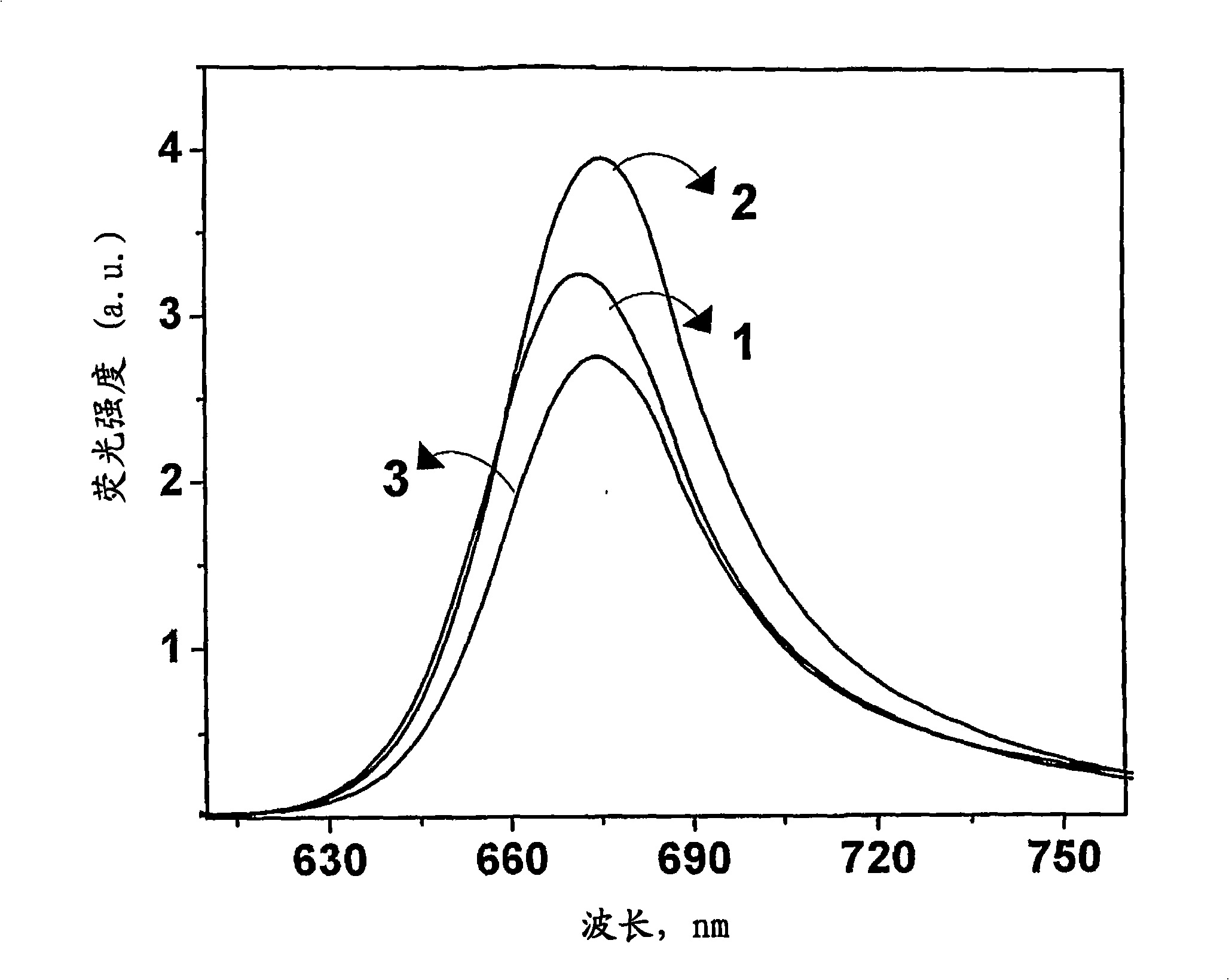
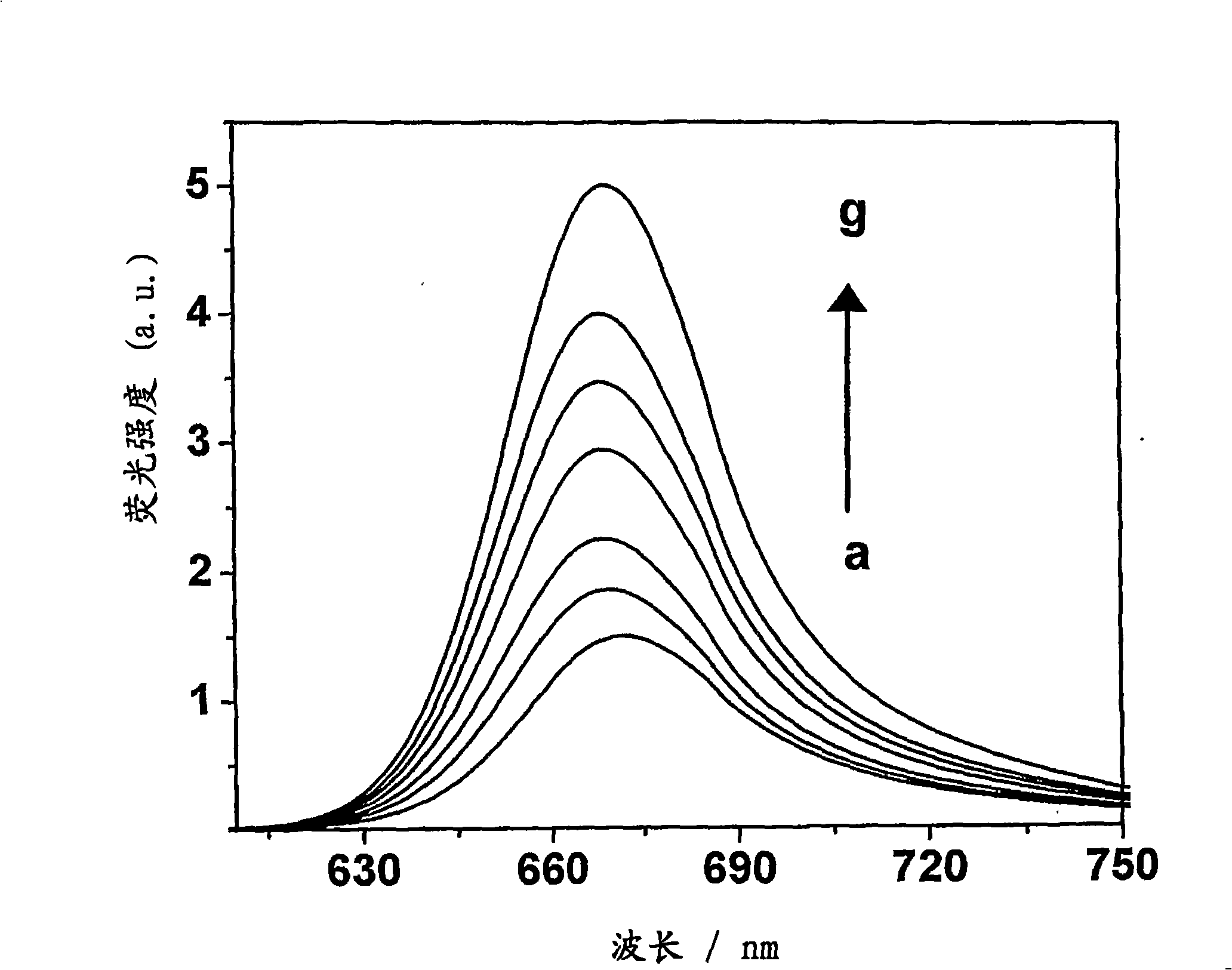
[0043] Formula 1 (wherein R 1 =-(CH 2 -CH 2 -O) n -CH 3 , n=4 and R 2 =-CH 3 ) preparation of squaraine dyes. N-methyl-N-(3,6,9,12-tetraoxatridecyl)aniline (400 mg, 1.35 mmol) and squaric acid (77 mg) in a mixture of n-butanol and benzene (1:3) , 0.67mmol) solution was refluxed by azeotropic distillation of water for 18h. The solvent was distilled off under reduced pressure, and the residue obtained was chromatographed on silica gel. Carry out column elution with the mixture (1:49) of methanol and chloroform to obtain 100mg (15%) general formula 1 (wherein R 1 =-(CH 2 -CH 2 -O) n -CH 3 , n=4 and R 2 =-CH 3 ) squarylium dye, mp 100-102°C; 1 H NMR (300MHz, CDCl 3 , 30°C, TMS): δ=3.21(s, 6H, -NCH 3 ), 3.37(s, 6H, -OCH 3 ), 3.72-3.52 (m, 32H, -OCH 2 ), 6.81 (d, 4H, J=8.96, Ar-H), 8.39 (d, 4H, J=8.95Hz, Ar-H); 13 C NMR (75MHz, CDCl 3 , 30°C, TMS): δ=39.68, 52.29, 58.97, 68.53, 70.42, 70.52, 70.56, 70.81, 71.83, 112.43, 119.90, 133.17, 154.41, 183.32, 188.58; IR...
Embodiment 2
[0045] Formula 2 (wherein R 1 , R 2 =-(CH 2 -CH 2 -O) n -CH 3 , n=4) preparation of squaraine dyes. Bis-(N,N-(3,6,9,12-tetraoxatridecyl))aniline (350 mg, 0.74 mmol) and squaric acid ( 42mg, 0.37mmol) solution was refluxed by azeotropic distillation of water for 18h. The solvent was distilled off under reduced pressure, and the residue obtained was chromatographed on silica gel. Carry out column elution with the mixture (1:99) of methanol and chloroform to obtain 40mg (5%) general formula 2 (wherein R 1 , R 2 =-(CH 2 -CH 2 -O) n -CH 3 , n=4) squaraine dye, mp 78-80°C; 1 H NMR (300MHz, CDCl 3 , 30°C, TMS): δ=3.37(s, 12H, -OCH 3 ), 3.37-3.55 (m, 64H, -OCH 2 ), 6.84 (d, 4H, J=8.96, Ar-H), 8.37 (d, 4H, J=8.95Hz, Ar-H); 13 C NMR (75MHz, CDCl 3 , 30°C, TMS): δ=50.73, 58.91, 68.31, 70.38, 70.49, 70.53, 71.80, 111.54, 115.85, 129.15, 147.60, 183.32, 188.58; IR (Neat): v max2918, 2867, 1610, 1584, 1114cm -1 ;Elemental analysis (%), C 52 h 84 N 2 o 18 Calcd for: C...
Embodiment 3
[0047] Formula 3 (wherein R 1 =-(CH 2 ) n -CO 2 X, n=3, X=H and R 2 =-CH 3 ) preparation of squaraine dyes. N-methyl-N-(carboxypropyl)aniline (319 mg, 1.74 mmol) and squaric acid (100 mg, 0.87 mmol) in a mixture of n-butanol and benzene (1:3) were refluxed by azeotropic distillation of water for 24 h . The solvent was distilled off under reduced pressure, and the residue obtained was chromatographed on silica gel. Column elution with a mixture of methanol and chloroform (1:9) afforded 100 mg (13%) of general formula 3 (where R 1 =-(CH 2 ) n -CO 2 X, n=3, X=H and R 2 =-CH 3 ) squaraine dye, mp 238-240°C (d); 1 H NMR (300MHz, [D 6 ]DMSO, 30°C, TMS): δ=1.91 (p, 4H, -CH 2 ), 2.40(t, 4H, J=7.2Hz, -CH 2 ), 2.91 (s, 6H, -NH 3 ), 3.35(t, 4H, J=7.3Hz, -CH 2 ), 6.99(d, 4H, J=9.07Hz, Ar-H), 8.05(d, 4H, J=8.97Hz, Ar-H); 13 C NMR (75MHz, [D 6 ]DMSO, 30° C., TMS): δ=21.82, 31.41, 38.46, 52.03, 112.64, 116.73, 129.17, 149.13, 179.23; IR (KBr): v max 3420, 2924, 1729, 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com