Derivatives of sulindac, use thereof and preparation thereof
A compound, CH3S technology, applied in the amide derivatives of sulindac, the preparation of the disclosed compounds, the treatment and prevention of precancerous conditions and cancers in mammals, can solve the problems of undescribed and undescribed anticancer activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
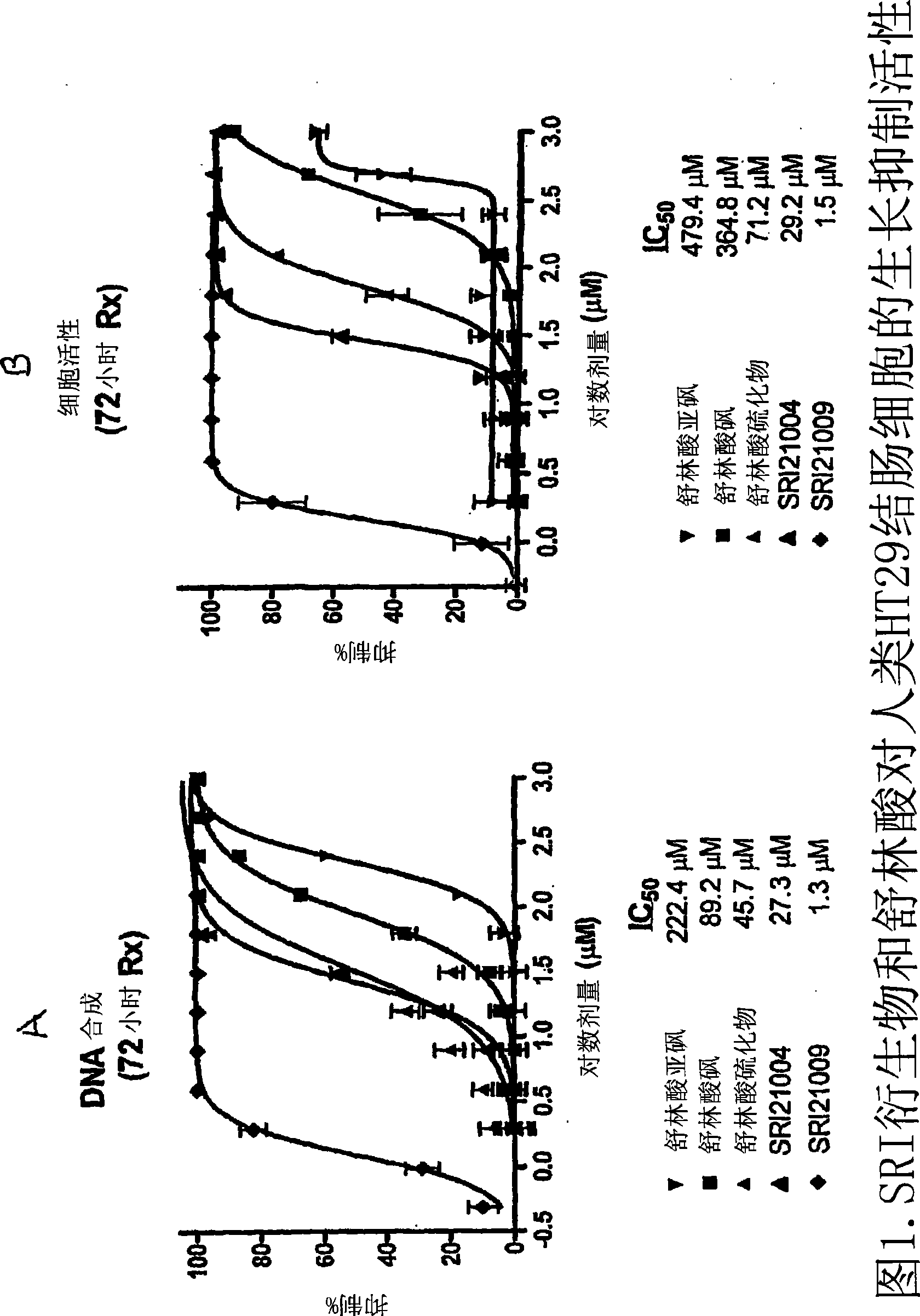
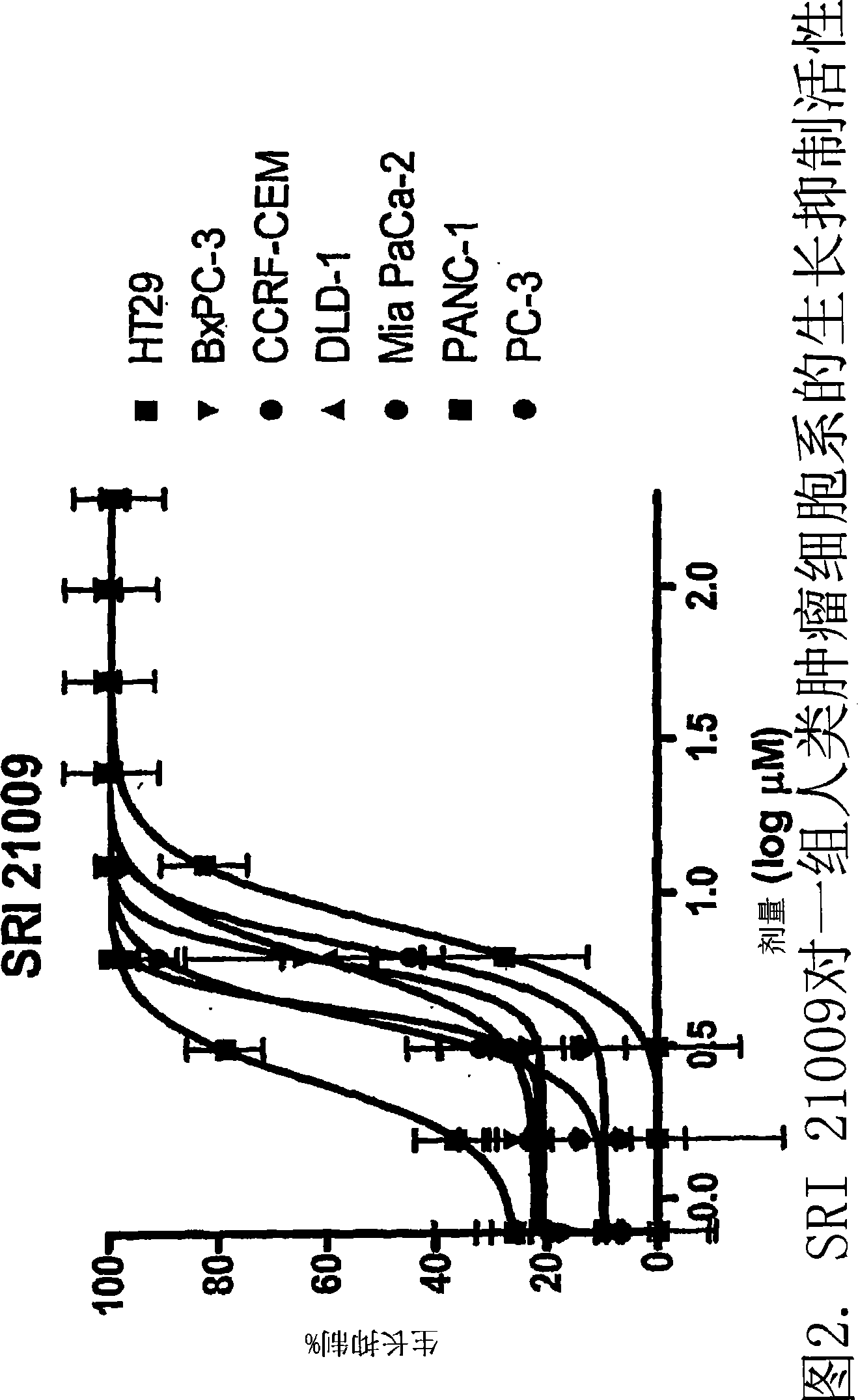
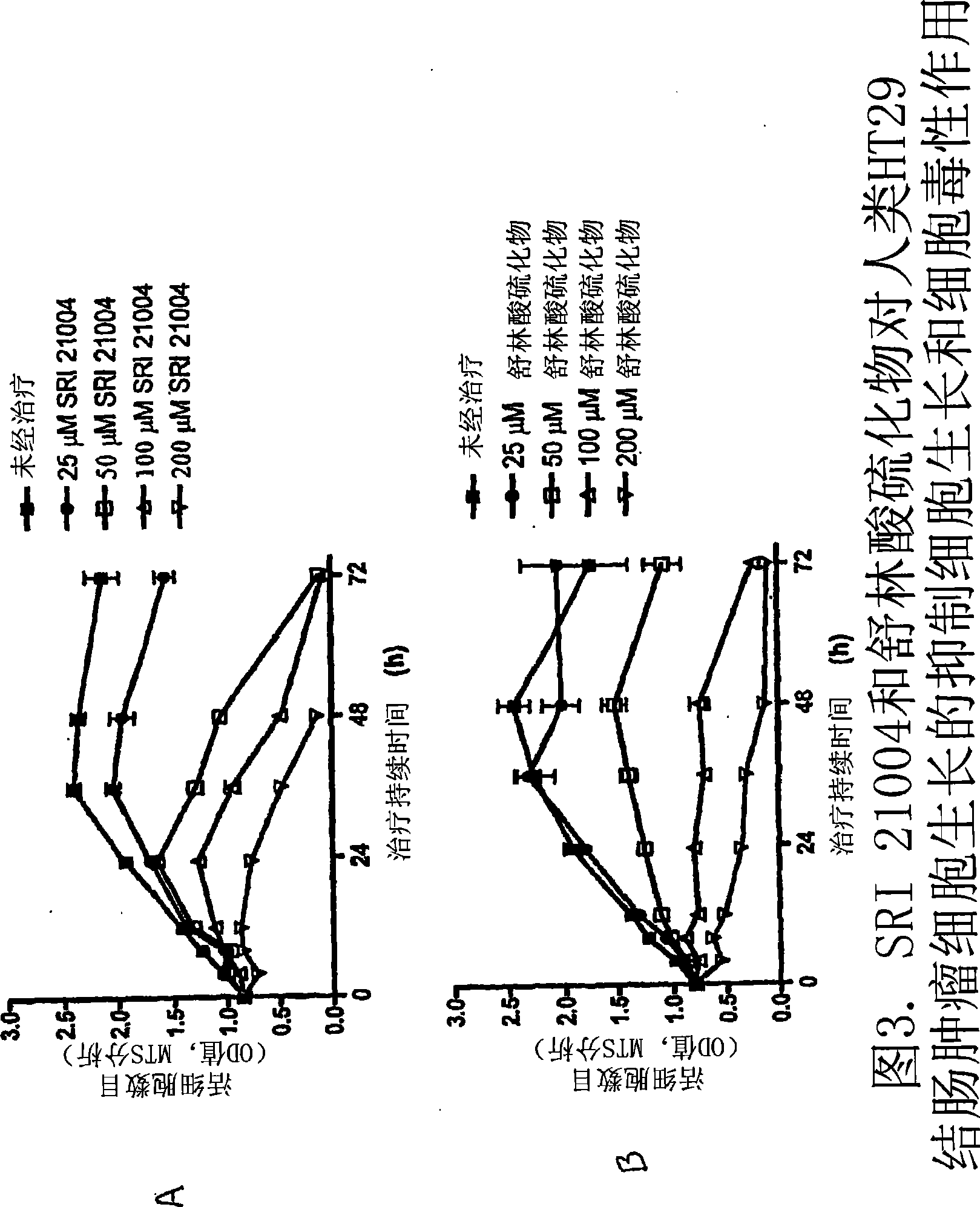
Image
Examples
example 1
[0065] N-[2-(Dimethylamino)ethyl]-5-fluoro-2-methyl-1-[[4-(methylsulfinyl)phenyl]methylene]-1H-indene-3 - Acetamide (1):
[0066] Use CHCl 3 / MeOH+0.2% NH 4 OH (9:1) to purify the crude product by column chromatography. The product was obtained as a yellow solid in 98% yield. ESI-MS m / z: 427[M+H] + . 1 H NMR (CDCl 3 ): δ 7.74-7.65 (2H, m, 2′-H, 3′-H), 7.17 (1H, dd, J=5.2Hz, 8.4Hz, 7-H), 6.88 (1H, dd, J=2.4 Hz, 8.9Hz, 4-H), 6.59 (1H, ddd, J = 2.4Hz, 9.0Hz, 11.0Hz, 6-H), 6.19 (1H, br s, -NHCH 2 CH 2 N(CH 3 ) 2), 3.28 (2H, dd, J = 5.7Hz, 11, 2Hz, -NHCH 2 CH 2 N(CH 3 ) 2 ), 3.51 (2H, s, 3-CH 2 ), 2.81 (3H, s, 4'-CH 3 ), 2.33 (2H, t, J=6.0Hz, -NHCH 2 CH 2 N(CH 3 ) 2 ), 2.13 (6H, s, N (CH 3 ) 2 , 2.12 (3H, s, 2-CH 3 ). Experimental values for CHN: C, 67.99; H, 6.45; N, 6.60. C 24 h 27 FN 2 o 2 Calculated for S: C, 67.59; H, 6.38; N, 6.57.
example 2
[0068] 5-fluoro-2-methyl-N-[(1-methyl-2-pyrrolidinyl)methyl]-1-[[4-(methylsulfinyl)phenyl]methylene]-1H -Indene-3-acetamide (2):
[0069] Use CHCl 3 / MeOH+0.2%NH 4 OH (8:1) to purify the crude product by column chromatography. Yellow solid, 91% yield. ESI-MS m / z: 467[M+H] + . 1 H NMR (DMSO-d 6 ): δ 8.10 (1H, t, J=4.2Hz, -NHCH 2 CH 2 ), 7.79 (1H, d, J=8.4Hz, 3′-H), 7.71 (1H, d, J=8.2Hz, 2′-H), 7.36 (1H, s, 8-H), 6.71 (1H , dd, J=5.4Hz, 8.5Hz, 7-H), 3.41 (2H, s, 1-CH 2 ), 3.12-3.06 (2H, m, -NHCH 2 CH 2 ), 2.88-2.79 (1H, m, 2"-H), 2.82 (3H, s, 4'-CH 3 ), 2.18 (3H, s, 2-CH 3 ), 2.12 (3H, s, N-CH 3 ), 2.00-1.91 (3H, m, 3"-H a , 5"-CH 2 ), 1.91-1.68 (2H, m, 3"-H b ), 1.68-1.50 (2H, m, -NHCH 2 CH b , 4"H b ), 1.40-1.07 (2H, m, -NHCH 2 CH a , 4"-H a ). Experimental values for CHN: C, 67.62; H, 6.48; N, 6.22. C 27 h 31 FN 2 o 2 S 0.8H 2 Calculated for O: C, 67.40; H, 6.83; N, 5.82.
example 3
[0071] 5-fluoro-2-methyl-1-[[4-(methylsulfinyl)phenyl]methylene]-N-[2-(1-piperazinyl)ethyl]-1H-indene- 3-Acetamide (3):
[0072] The crude product was subjected to column chromatography using CHCl 3 / MeOH+0.2% NH 4 OH (4:1) and purified by prep TLC using CHCl 3 / MeOH+0.2% NH 4 OH (5:1) for further purification. Yellow solid, 58% yield. ESI-MS m / z: 468[M+H] + . 1 H NMR (DMSO-d 6 ): δ 7.97 (1H, t, J=4.2Hz, -NHCH 2 CH 2 ), 7.80 (2H, d, J=8.4Hz, 3′-H, 5′-H), 7.72 (2H, d, J=8.2Hz, 2′-H, 6′-H), 7.35 (1H, s, 8-H), 7.15 (1H, dd, J = 5.3Hz, 8.4Hz, 7'-H), 7.10 (1H, dd, J = 2.4Hz, 9.3Hz, 4-H), 6.71 (1H, ddd, J=2.4Hz, 9.6Hz, 11.8Hz, 6-H), 3.43 (2H, s, 1-CH 2 ), 3.16 (2H, dd, J = 5.94Hz, 11.9Hz, -NHCH 2 CH 2 ), 2.66 (4H, t, J=4.7Hz, 3"-H and 5"-H), 2.51-2.30 (6H, m, -NHCH 2 CH 2 , 2"-H and 6"-H), 2.18 (3H, s, 2-CH 3 ). Experimental values for CHN: C, 63.53; H, 6.46; N, 8.53. C 26 h 30 FN 3 o 2 S 1.3H 2 Calculated for O: C, 63.60; H, 6.69; N, 8.55.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com