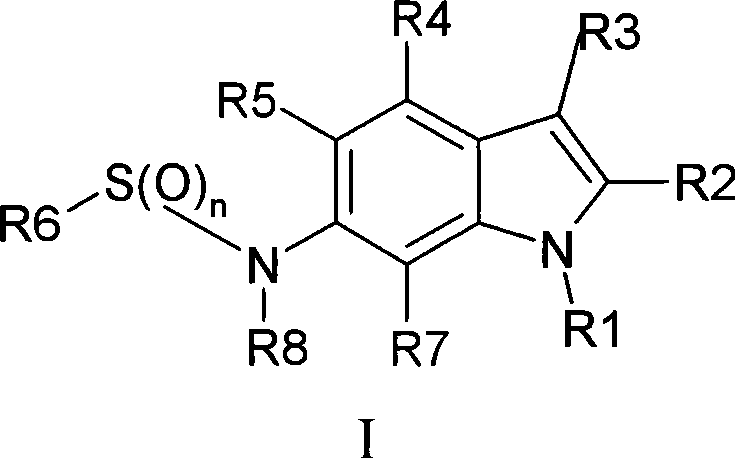
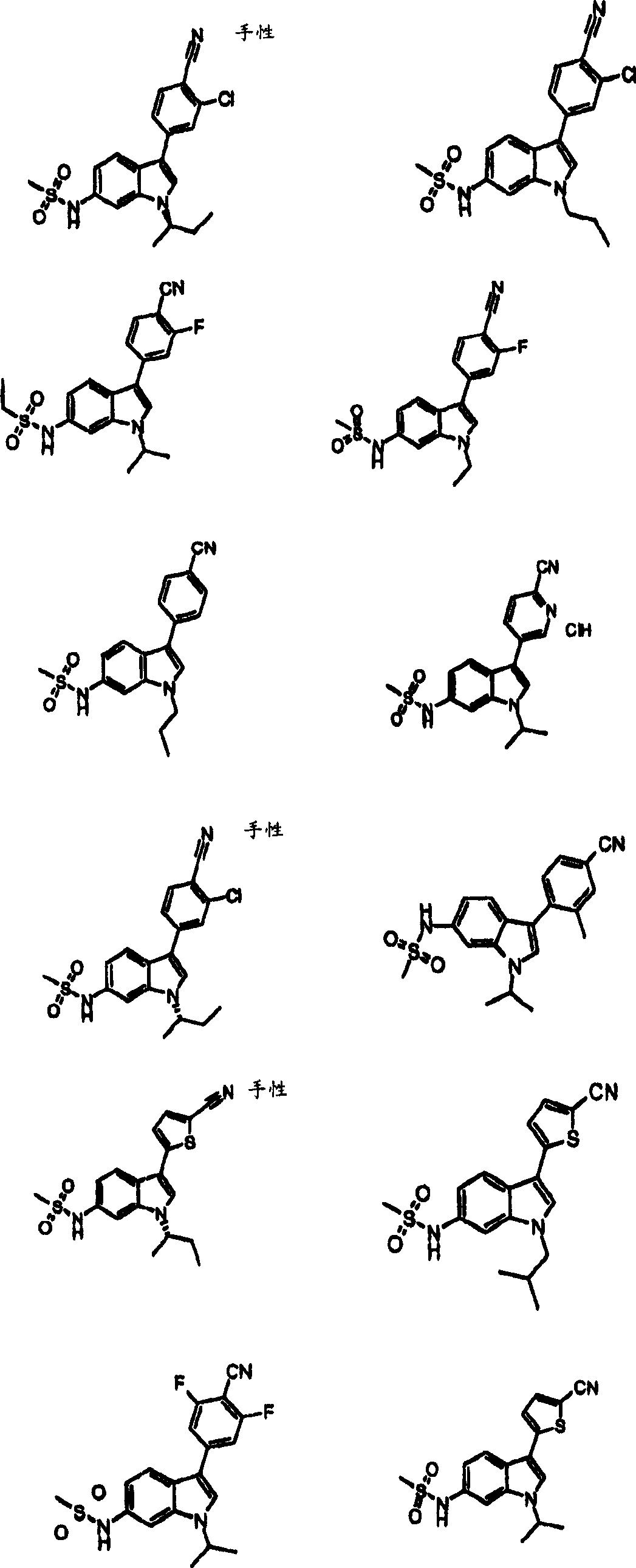
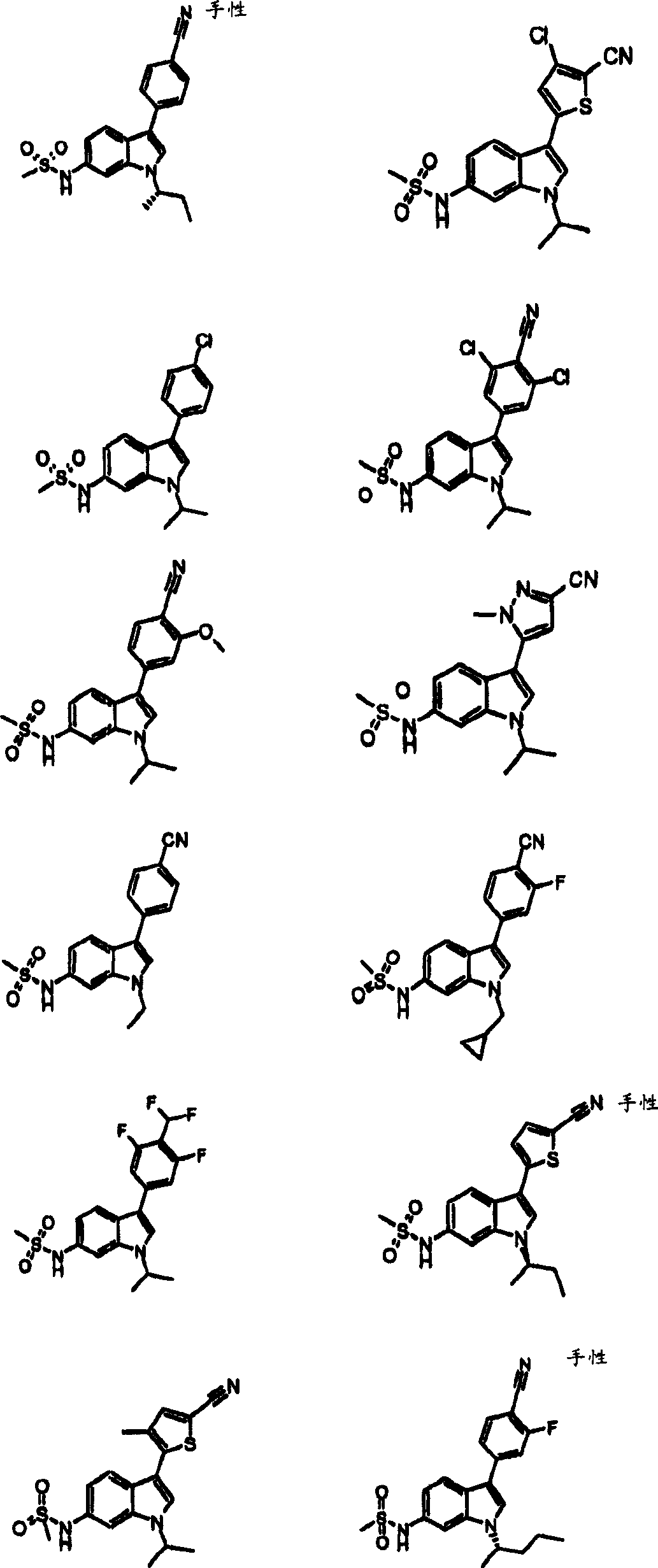
Indole sulfonamide modulators of progesterone receptors
一种烷基、C1-C6的技术,应用在含有效成分的医用配制品、药物组合、性疾病等方向,能够解决没有描述有效治疗孕酮受体配体、子宫内膜异位症有效性有效性低、有效性低等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 178
[0175] Example 178 N-[3-(6-cyano-5-fluoro-pyridin-3-yl)-1-isopropyl-1H-indol-6-yl]-methanesulfonamide
[0176] Method X will N 2 Bubble through N-[1-isopropyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-1H- Indol-6-yl]-methanesulfonamide (0.186g, 0.493mmol), 5-bromo-2-cyano-3-fluoropyridine (0.090g, 0.448mmol), K 3 PO 4 (1.3M) (0.60ml), and a mixture of dioxane (1.2ml) for 5 minutes. Add tricyclohexylphosphine (3.0 mg, 0.011 mmol) and Pd 2 (dba) 3 (4.1 mg, 0.0045 mmol). The tube was sealed and stirred at 100°C for 18 hours. After cooling to room temperature, extract with EtOAc and wash with NaHCO 3 washing. MgSO for organic phase 4 dry. The crude residue was purified using silica gel chromatography to afford 0.112 g (67% yield) of the title compound, MS: 373.0 (M+H)
[0177] N-[3-(3,5-Difluoro-4-hydroxymethyl-phenyl)-1-isopropyl-1H-indol-6-yl]-methanesulfonamide
[0178] Method S: Weigh N-(3-bromo-1-isopropyl-1H-indol-6-yl)-methanesulfonamide (100mg, 0.302mmol...
Embodiment 289
[0290] Example 289 N-[3-(5-cyano-4-fluoro-thiophen-2-yl)-1-isopropyl-1H-indol-6-yl]-methanesulfonamide
[0291]N-[3-(4-Chloro-5-cyano-thiophen-2-yl)-1-isopropyl-1H-indol-6-yl]-methanesulfonamide (Example 118 ) (0.15g, 0.381mmol) and CsF (0.324g, 2.13mmol) in DMSO (5ml) was heated at 150°C for 6 hours. The mixture was cooled to 21 °C and diluted with EtOAc. The mixture was washed with water, brine, and MgSO 4 dry. The material was filtered and concentrated to dryness. The crude product was purified by reverse phase chromatography to afford 5 mg of the title compound. MS:378.0(MH+)
Embodiment 290
[0292] Example 290 2-fluoro-4-(1-isopropyl-6-methanesulfonylamino-1H-indol-3-yl)-thiobenzamide:
[0293] Add one drop of di-isopropylethylamine and one drop of water to the solution containing N-[3-(4-cyano-3-fluoro-phenyl)-1-isopropyl-1H-indol-6-yl] - Methanesulfonamide (100 mg, 0.27 mmol) in 10 mL of dimethoxyether. Heat to reflux, then add O,O'-diethyl dithiophosphate (151 mg, 0.81 mmol) and reflux overnight in a sealed tube. The solvent was evaporated. The residue was purified using silica gel chromatography eluting with a gradient of EtOAc and hexanes to afford 99 mg (91%) of the desired product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com