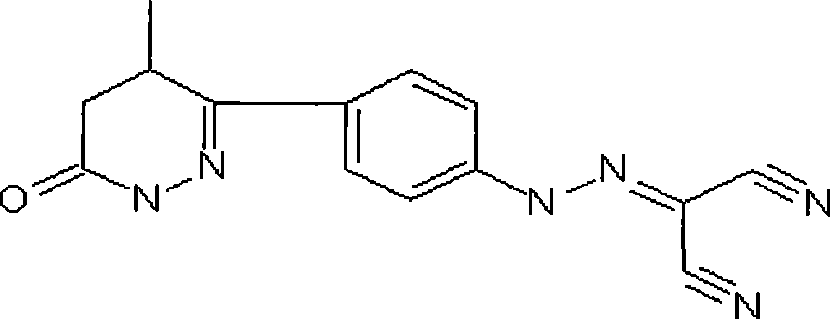
Stable Levosimendan pharmaceutical composition and preparation method thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as time-consuming, unresolved substantive problems, inconvenient storage and transportation, etc., and achieve the effects of simple preparation process, convenient transportation, and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
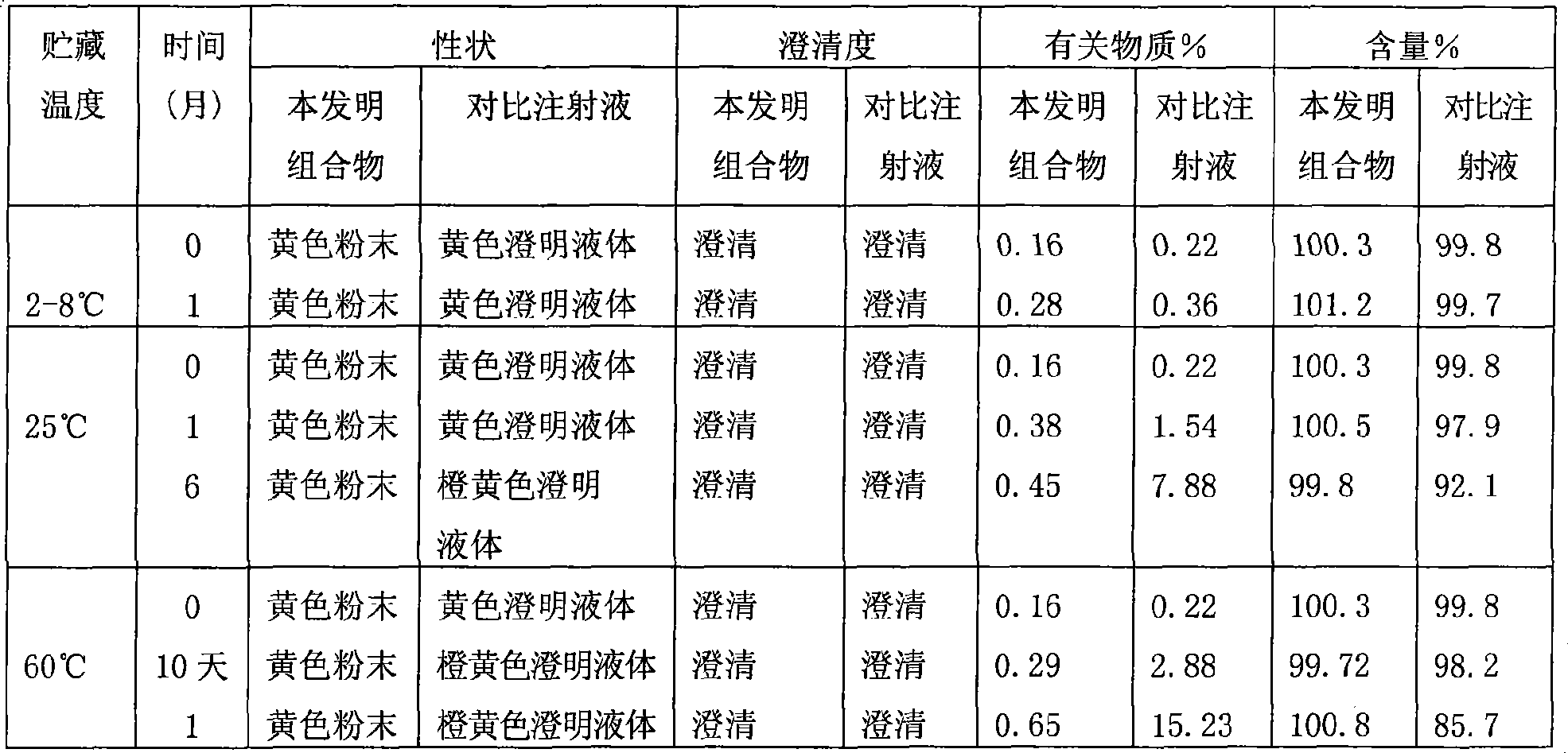
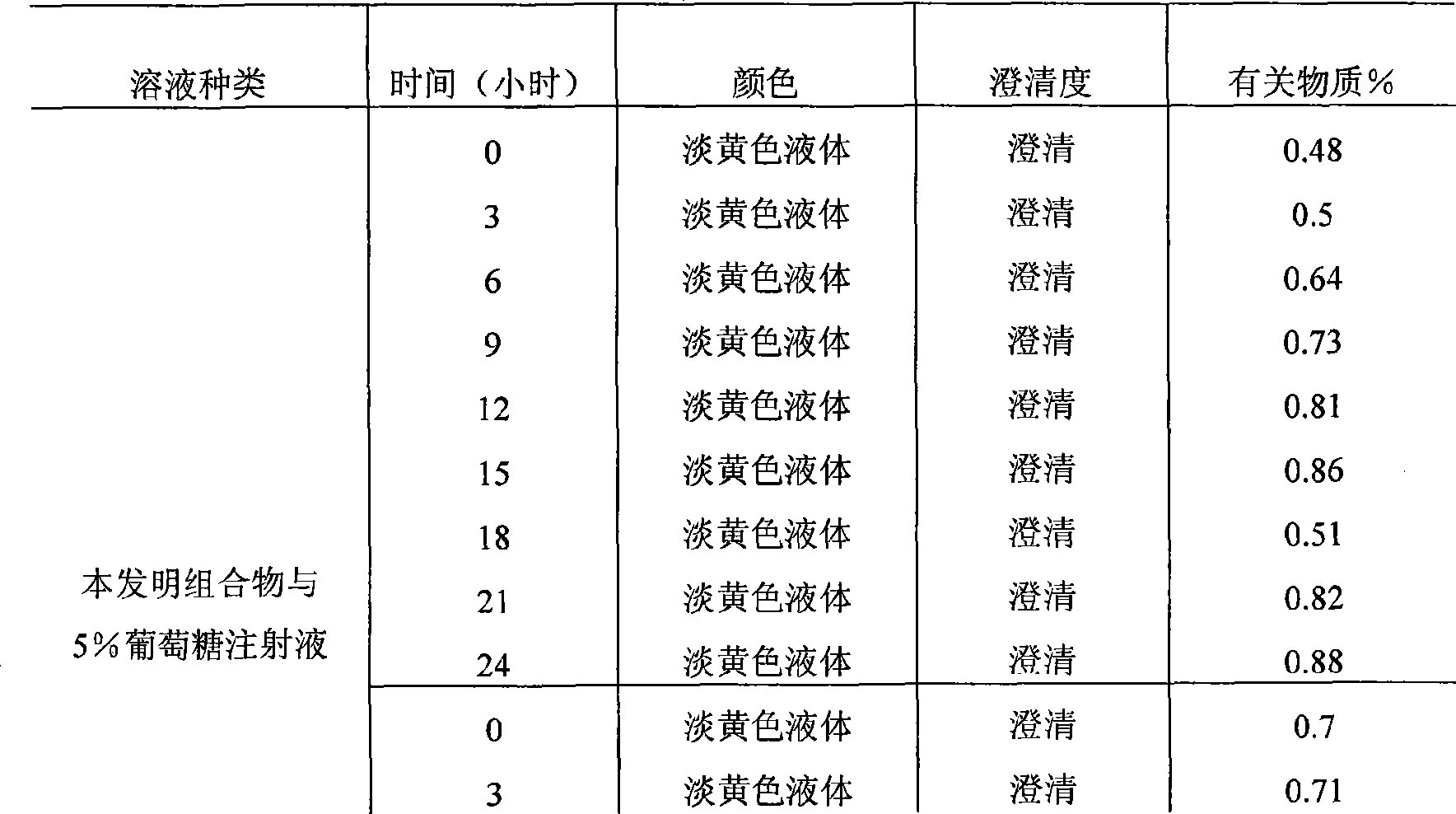
[0041] Accurately weigh 12.5g of levosimendan sterile powder (calculated as 1000 tubes), pack into 7ml vials, each 12.5mg; weigh 250g of hydroxypropyl beta cyclodextrin, add to 4500ml under stirring In absolute ethanol, continue to stir until the solution is clear, and set the volume to 5000ml; add 0.05% activated carbon for adsorption for 15min, decarbonize and filter, sterilize and filter with a 0.22um microporous membrane, and then pack into vials, 5ml each. The vials of the above-mentioned sterile powder and solvent for injection are covered with rubber stoppers, pressed with aluminum caps, and packaged to obtain the product.
Embodiment 2
[0043] Accurately weigh 6.25g of levosimendan sterile powder (calculated according to 1000 tubes), and pack it into 7ml vials, 6.25mg per tube; weigh 150g of hydroxypropyl beta cyclodextrin, add it to 2000ml under stirring In absolute ethanol, continue to stir until the solution is clear, and set the volume to 2500ml; add 0.05% activated carbon to absorb for 15min, decarbonize and filter, sterilize and filter with a 0.22um microporous membrane filter, and then pack into vials, 2.5ml each . The vials of the above-mentioned sterile powder and solvent for injection are covered with rubber stoppers, pressed with aluminum caps, and packaged to obtain the product.
Embodiment 3
[0045] Accurately weigh 25g of levosimendan sterile powder (calculated according to 1000 tubes), pack into 15ml vials, 25g per tube; weigh 650g of hydroxypropyl beta-cyclodextrin, add to 11000ml anhydrous In ethanol, continue to stir until the solution is clear, and set the volume to 12000ml; add 0.05% activated carbon to absorb for 15min, decarbonize and filter, sterilize and filter with a 0.22um microporous membrane, and then pack into vials, each 12ml. The vials of the above-mentioned sterile powder and solvent for injection are covered with rubber stoppers, pressed with aluminum caps, and packaged to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com