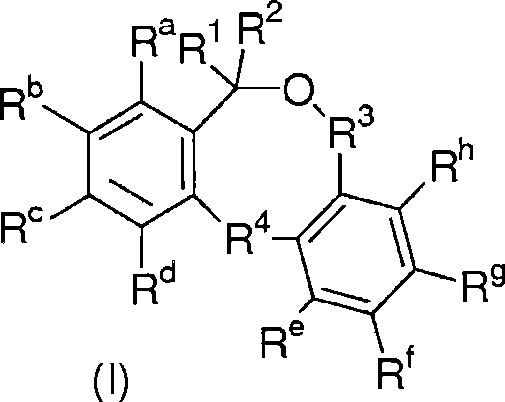
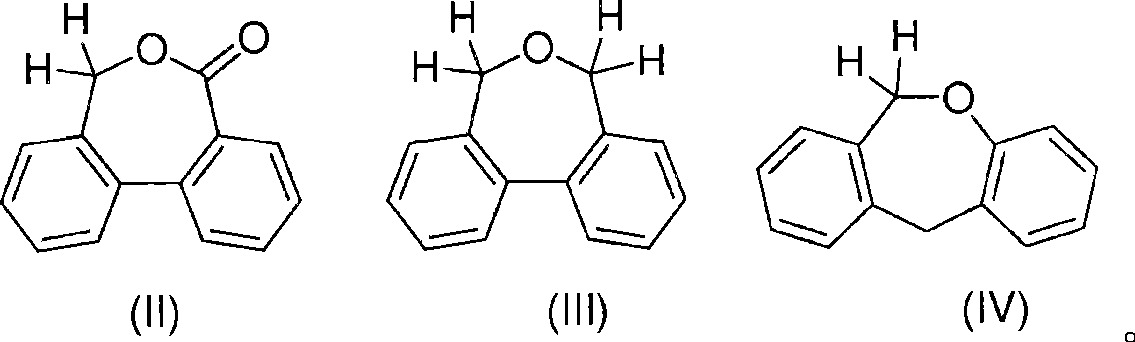
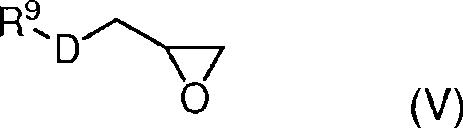
Low shrinkage epoxy-cationic curable compositions
A technology of cationic compound and epoxy composition, applied in the field of low-shrinkage epoxy cationic curable composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0138] Preparation of Curable Formulations
[0139] Bis F-DGE (8.00 g, 25.6 mmol, amount of epoxy groups = 51.2 mmol), DBOX (2.52 g, 12.0 mmol) and tetraphenylethylene glycol (25 mg) were mixed at 110° C. and removed under vacuum. gas to obtain a homogeneous mixture. After cooling to room temperature, HD7980 (diaryliodonium hexafluorophosphate, 50 mg) was added to the mixture to give the corresponding curable formulation A as a homogeneous liquid.
[0140]
[0141] Curing Reaction and Shrinkage Test
[0142] About 5 g of mixture A obtained was used to measure its volume by gas-pychnometer. From the weight and volume of the sample, calculate its uncured density (=D 固化前 ). Three independent samples were used for the test, and each sample was tested 5 times to calculate the average density (D 固化前 ). Calculated D 固化前 It is 1.216. Then, the mixture was transferred into a silicone rubber mold and cured at 110°C for 2 hours, followed by post-curing at 140°C for 1 hour to o...
Embodiment 1-1-a
[0147] Preparation of Curable Formulations
[0148] Powdered DBOX (2.00 g, 9.51 mmol) was dispersed in Bis F-DGE (8.00 g, 25.6 mmol, amount of epoxy groups = 51.2 mmol). Tetraphenylethylene glycol (25 mg) and HD7980 (diaryliodonium hexafluorophosphate, 50 mg) were added to the resulting DBOX / BisF-DGE dispersion to obtain a heterogeneous preparation, which was used in Cured under the same conditions as Example 1-1.
[0149]
[0150] Curing reaction and shrinkage test were performed in the same manner as in Example 1-1.
[0151] As shown in Table 1, there is no difference in the degree of shrinkage between Example 1-1-a and Example 1-1, which shows that the addition of powdered DBOX that is not dissolved in the epoxy resin is equally effective in inhibiting their volume shrinkage.
Embodiment 1-1-b
[0153] Preparation of Curable Formulations
[0154] Powdered DBOX (2.00 g, 9.51 mmol) and silica filler FL1966 (10.0 g) were dispersed in F-DGE (8.00 g, 25.6 mmol, amount of epoxy groups = 51.2 mmol), tetraphenylethylene glycol (25 mg ) and HD7980 (diaryliodonium hexafluorophosphate, 50mg) in a mixture. The resulting heterogeneous formulation was cured under the same conditions as used in Example 1-1.
[0155]
[0156] Curing reaction and shrinkage test were performed in the same manner as in Example 1-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com