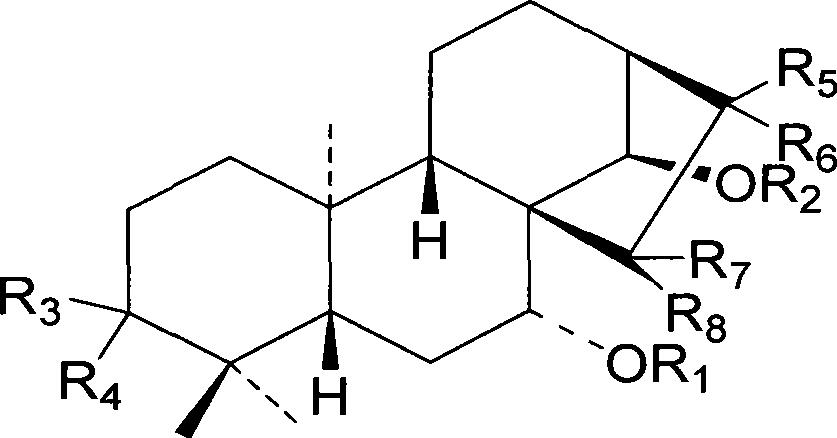
Use of diterpenoids from Isodon japonica var.galaucocalyx in preparing anti-cancer medicine
A technology of compound and diterpenes, applied in the field of preparation of anticancer drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
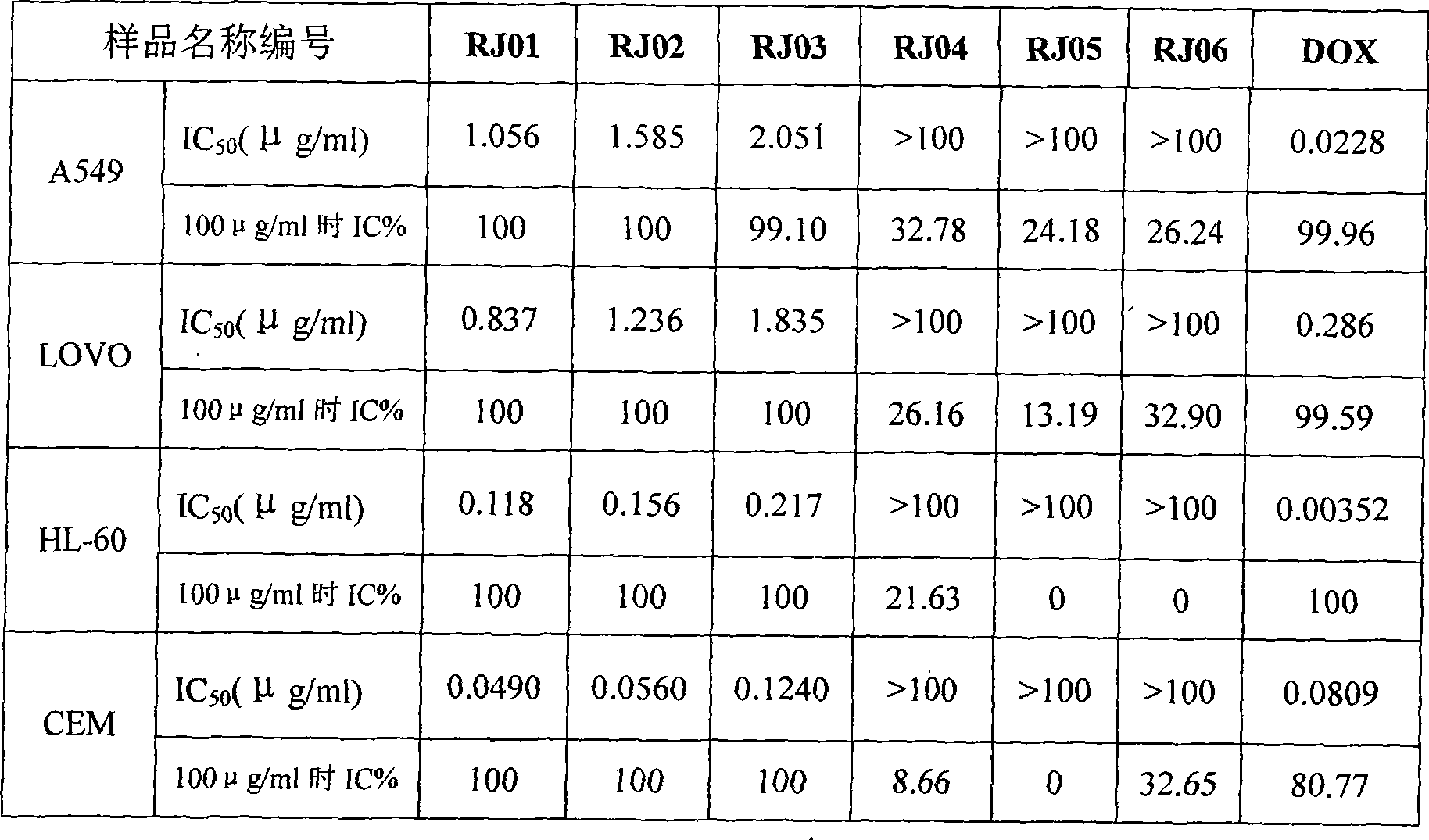
[0017] The present invention will be described in detail below through in vitro anti-tumor experiments.
[0018] In Vitro Antitumor Activity Test of Elyculin A, Elyculin C, Elyculin D, Elyculin, Elyculin
[0019] 1. Experimental drug:
[0020] R. calyxin A (RJ01), R. calyxin B (RJ02), R. calyxin C (RJ04), R. calyxin D (RJ03), R. calyxin (RJ05), R. calyxin (RJ06) from R. It is isolated from fragrant tea vegetables, and the preparation method is detailed in [Zhao Bao Xiang (xiang Zhaobao), Hai Sheng Chen (陈海生), et al.Two new diterpenoids from Rabdosia japonica var. glaucocalyx, Chinese Chemical Letter 2008; 19:852- 854; Zhao Bao Xiang (xiang Zhaobao), Hai Sheng Chen (Chen Haisheng), et al. Diterpenoids from Rabdosiajaponica Asian Journal of Chemistry 2009; 21:3].
[0021] Control drug: doxycycline (DOX), Shijiazhuang Ruitian Biochemical Co., Ltd.
[0022] 2. Cell line: provided by the Pharmacology Laboratory of Shanghai Institute of Pharmaceutical Industry
[0023] ①A549 (hu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com