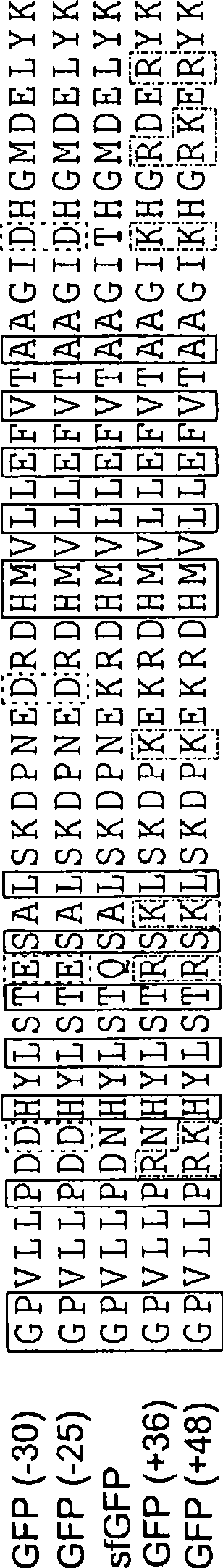Protein surface remodeling
A protein, green fluorescent protein technology, applied in the field of protein surface reconstruction, can solve problems such as aggregation troubles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
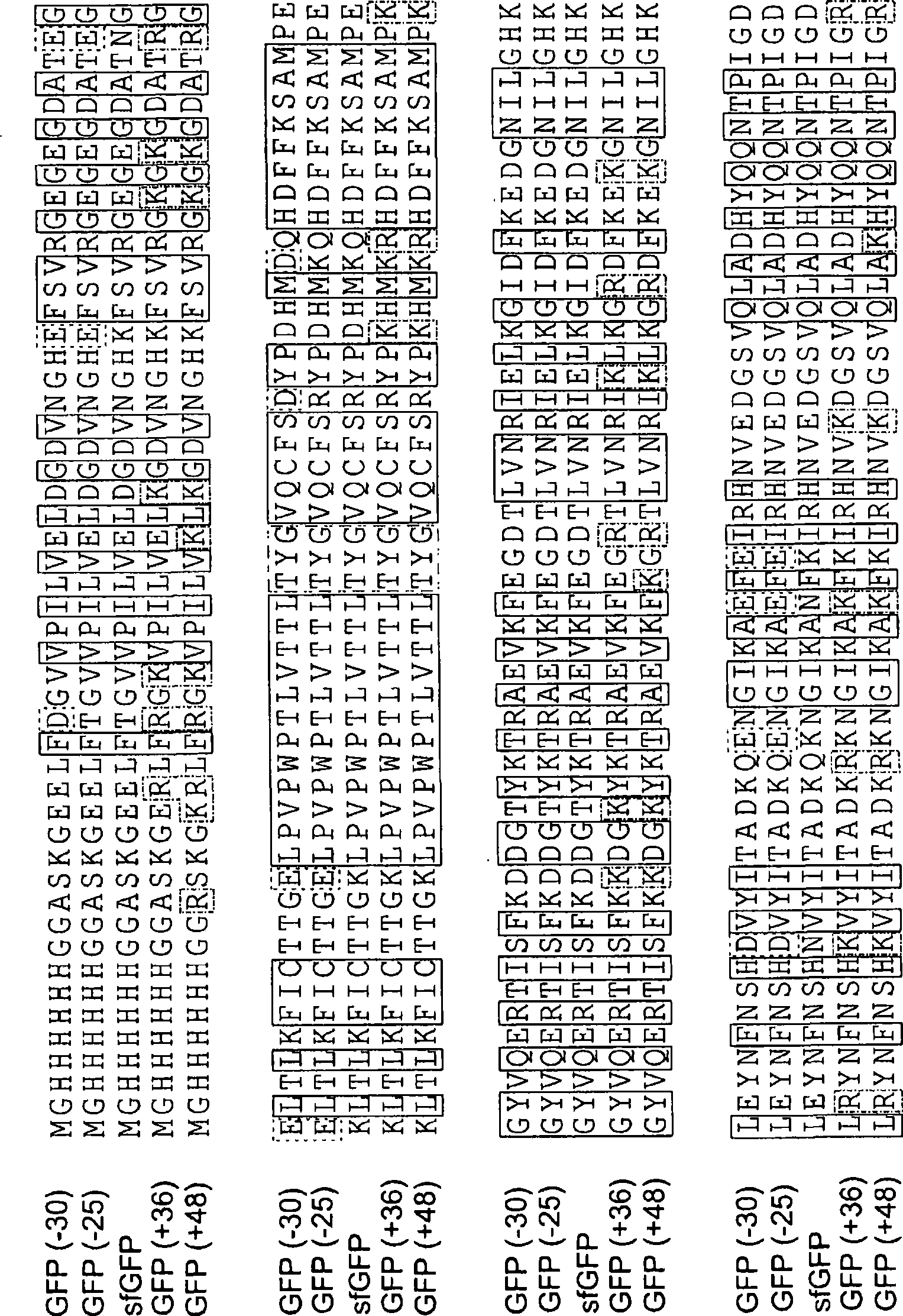
[0269] Example 1 - Supercharging Proteins Can Confer Great Resilience (Resilience)
[0270] Protein aggregation is a well-known cause of human disease (Cohen, F.E.; Kelly, J.W., Nature 2003, 426, (6968), 905-9; Chiti, F. ); Dobson (Dobson, C.M), Annu Rev Biochem 2006, 75, 333-66; each incorporated herein by reference), are also major challenges facing the use of proteins as therapeutic or diagnostic agents. Problems (Frokjaer, S.; Otzen, D.E., Nature Reviews: Nat Rev Drug Discov 2005, 4, (4), 298-306; Fowler, S.B.); Poon, S.; Muff, R.; Chiti, F.; Dobson, C.M.; Zurdo, J., National Academy of Sciences Proc. (Proc Natl Acad Sci USA) 2005, 102, (29), 10105-10; each incorporated herein by reference). Knowledge of the problem of protein aggregation has been gained from the study of natural proteins. We already know that proteins are least soluble when they carry an isoelectric point of zero net charge (Loeb, J., J Gen Physiol 1921, 4, 547-555, incorporated herein by reference mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com