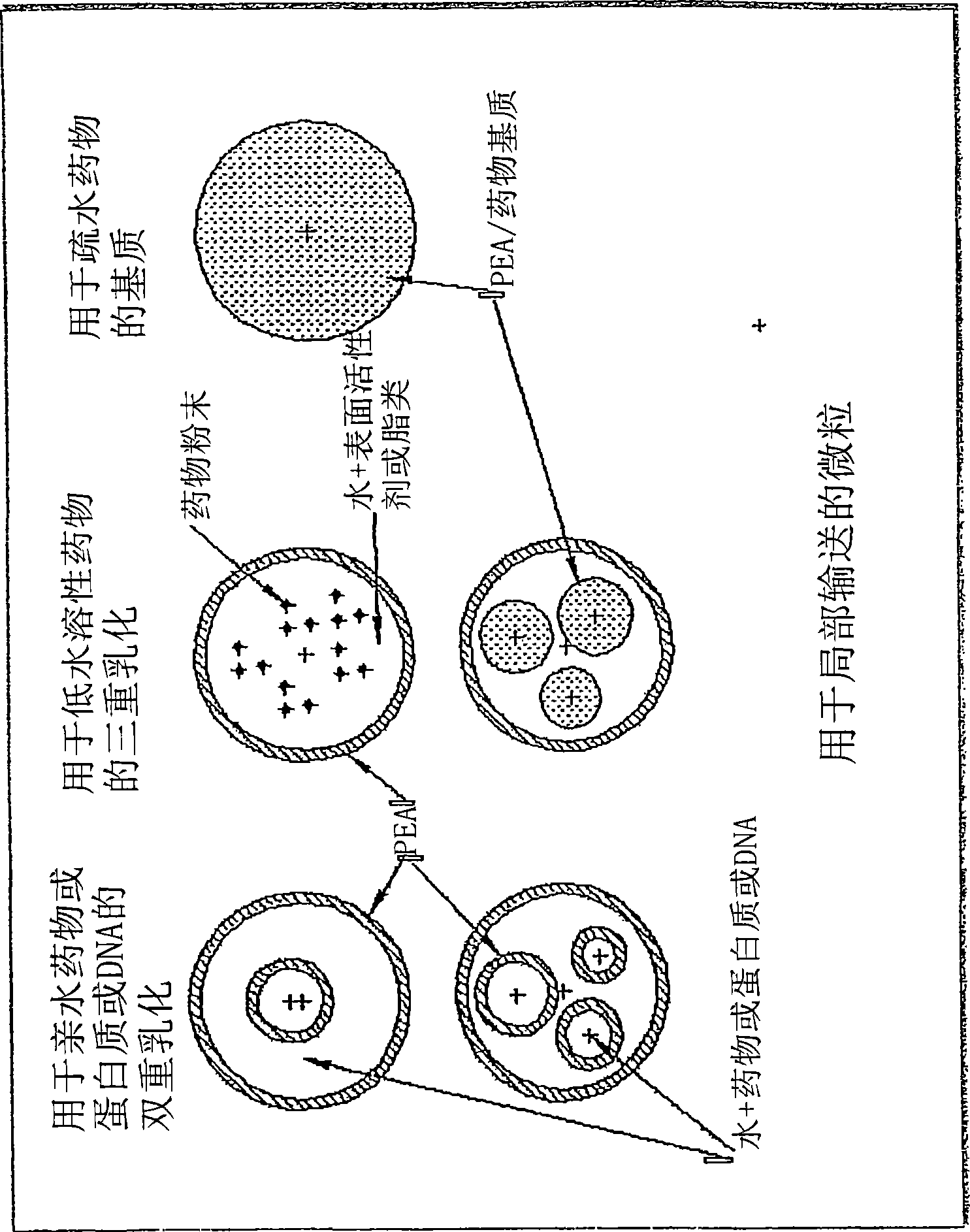
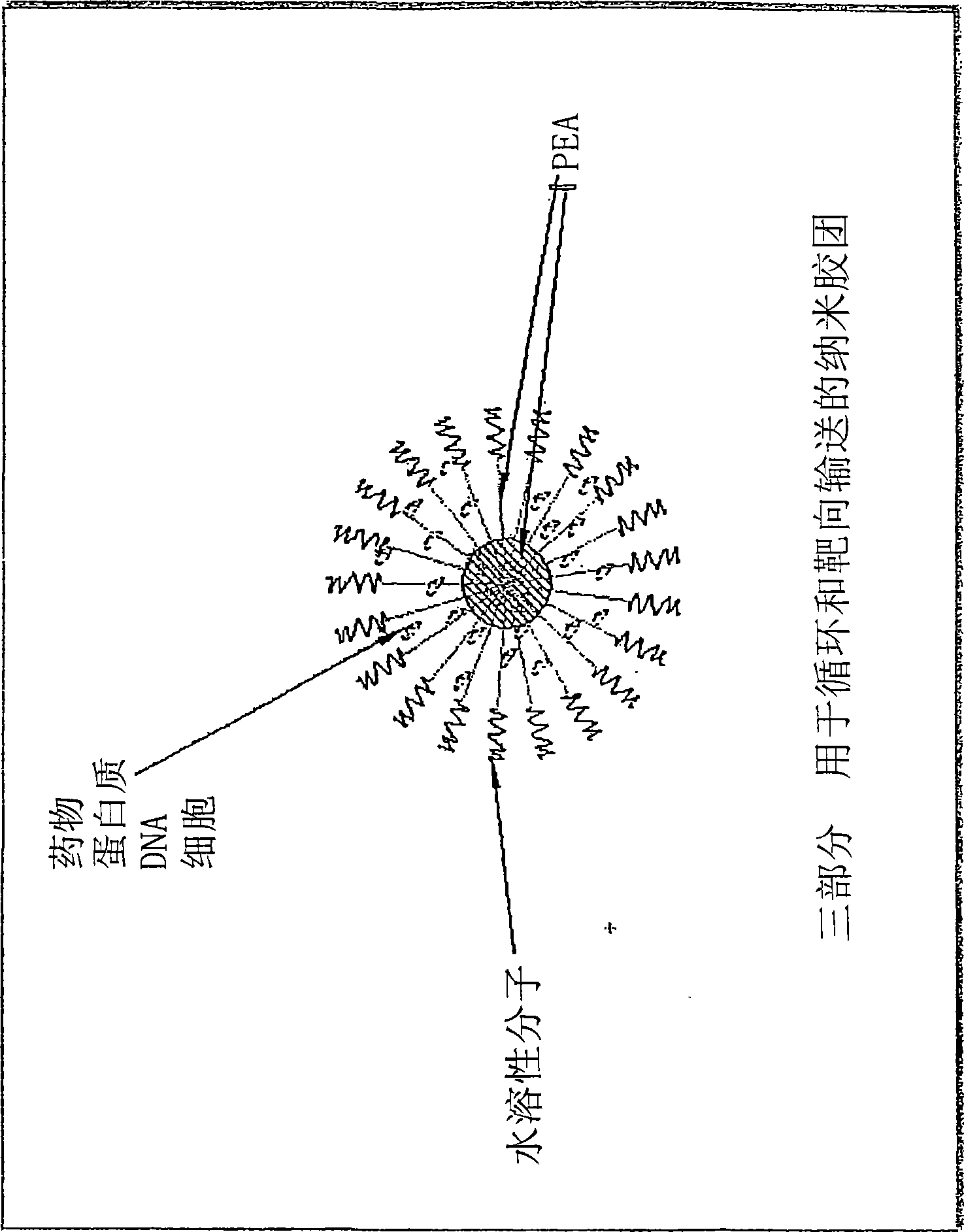
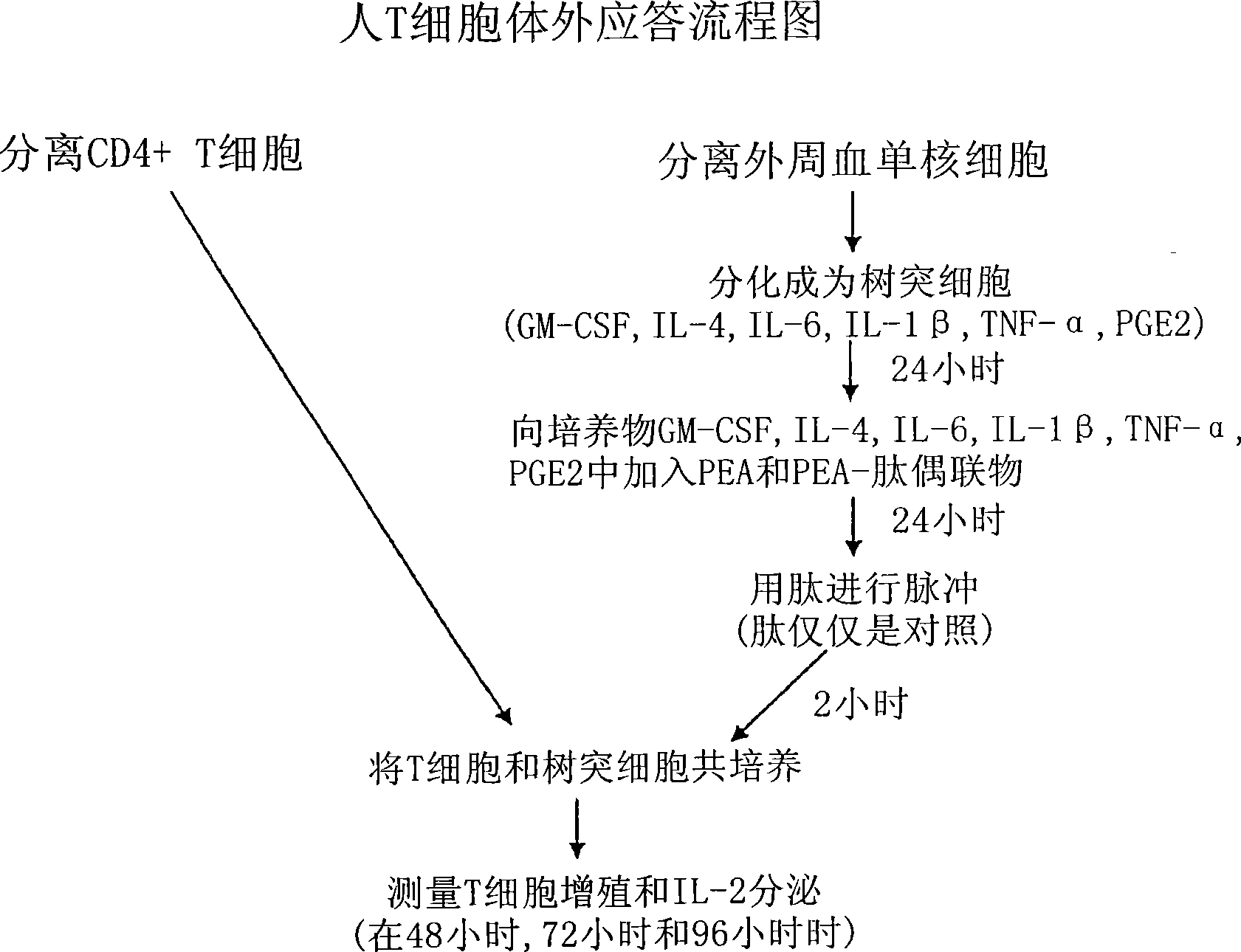
Vaccine delivery compositions and methods of use
A composition and vaccine technology, applied in the field of allelic vaccine delivery compositions, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0296] Synthesis of PEA-antigen conjugates
[0297] [0176] Synthesis of PE4 succinimidyl ester (PEA-Osu). All examples are from N-acetylated polymer (A). PEA 1.392g, 754 μ M, calculated molecular weight MW=1845 per repeating unit (formula I, R 1 =(CH 2 ) 8 ; 2 = H; R 3 =(CH 3 ) 2 CHCH 2 ; 4 =(CH 2 ) 6 ; n=70; m / m+p=0.75 and p / m+p=0.25), which were dissolved in 7 ml of anhydrous DMF under stirring. To this slightly viscous PEA solution was added 0.110 g, 955 [mu]M solid N-hydroxysuccinimide (NHS). 146 mg, 759.8 [mu]M of 1-ethyl-3-(3'-dimethylaminopropyl)carbodiimide hydrochloride was transferred as a suspension in DMF. The total volume of DMF used for this reaction was 10 ml. This reaction was carried out at room temperature under a nitrogen atmosphere for 24 hours.
[0298] Synthesis of PEA-influenza peptide conjugates:
[0299] [0177] B1) With a 49.5 μM aliquot of the activated ester (A) in DMF, and 96 mg (49.5 μM) H-PKYVKQNTLKLAT-OH, as the trifluoroacetate s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com