Vaccines against chlamydial infection
A Chlamydia trachomatis, immunogenicity technology, applied in the direction of Chlamydia antigen components, vaccines, antibody medical components, etc., can solve the problems such as no special public treatment of eye infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
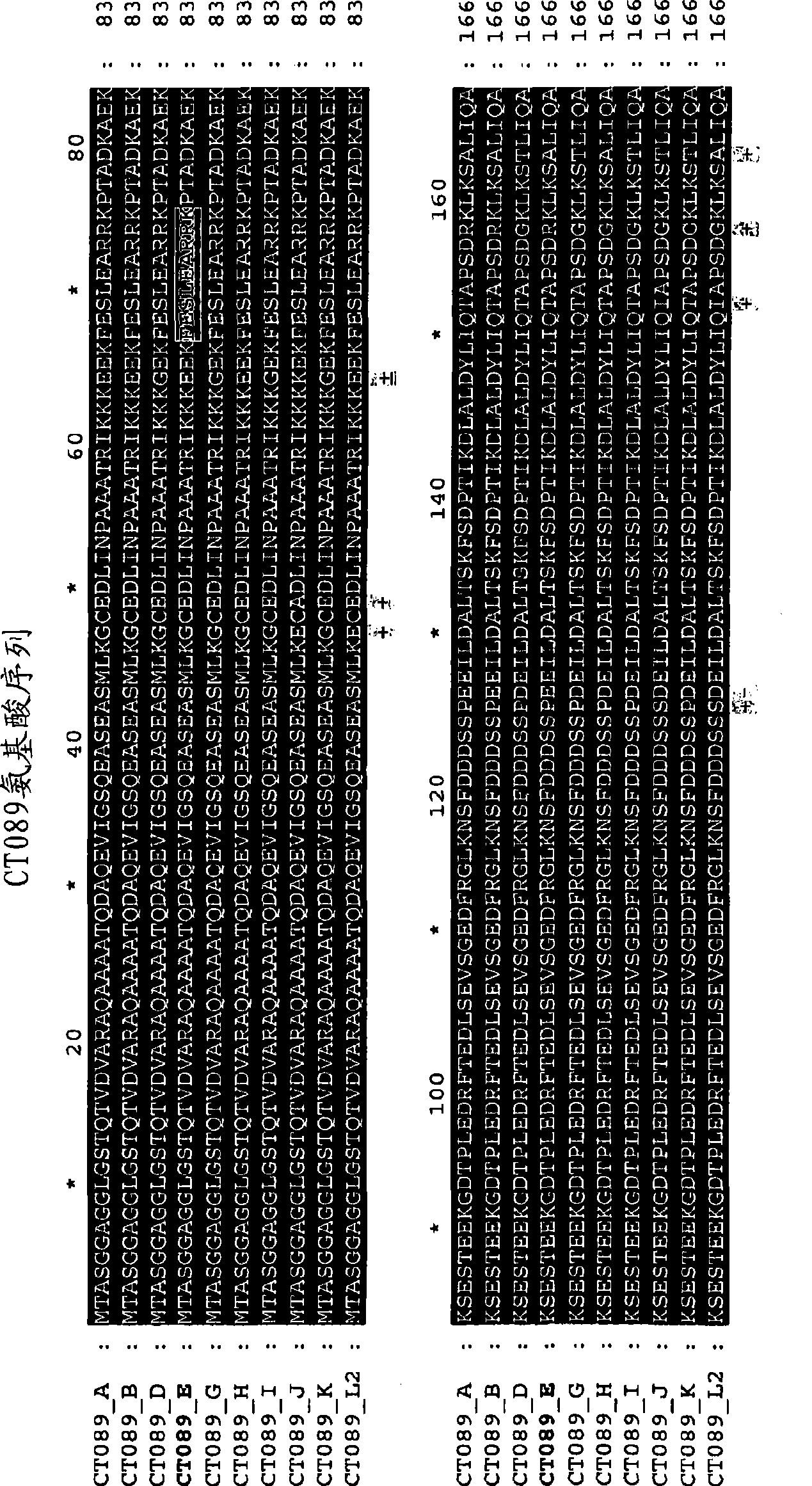
[0395] Example 1: Sequence comparison of Ct-089, Ct-858 and Ct-875
[0396] Chlamydia trachomatis serotype E is a common ocular serotype and was chosen as the basis for other sequence comparisons.
[0397] Multiple alignments of amino acid sequences for comparison have been performed using the CLUSTAL W program available from the Lasergene software package, version 5.0 (marketed by DNASTAR, Inc., Madison, WI). The basic multiple alignment algorithm involves a three-step process: all sequence pairs are aligned separately to compute a distance matrix that yields the divergence of each pair of sequences, then a guide tree is computed from the distance matrix and finally the sequences are progressively aligned according to the guide tree. The CLUSTAL W algorithm is described in Thompson et al., Nuc. Acids Res. 22:4673-4680 (1994). The alignment is shown in Figure 1, 2a / 2b and 3a / 3b.
[0398] T-helper epitopes are peptides that bind HLA class II molecules and are recognized by T-...
Embodiment 2
[0399] Example 2: Eliciting a protective immune response against Chlamydia trachomatis infection in mice
[0400] Experiment overview
[0401] Female C57BL / 6 and C3H mice were inoculated with a combination of Ct-089, Ct-858 and Ct-875 proteins from serotype E formulated in adjuvant (two or three intramuscular immunizations, using two different dose level). Positive controls were vaccinated with UV-attenuated protomers from serotype A or K in adjuvant. A negative control group was vaccinated with adjuvant only.
[0402] Mice were infected by individual eye challenges with ocular serotypes A, B or ocular vaginal serotype K. The course of infection was monitored by using eye swabs.
[0403] method
[0404] test individual
[0405] 240, 6-week-old female mice (consisting of 144 C3H mice and 96 C57BL / 6 mice) were obtained from Charles River Laboratories (Wilmington, Massachusetts). Animals were divided into 30 groups of 8 mice each (18 groups of C3H mice and 12 groups of C57...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com