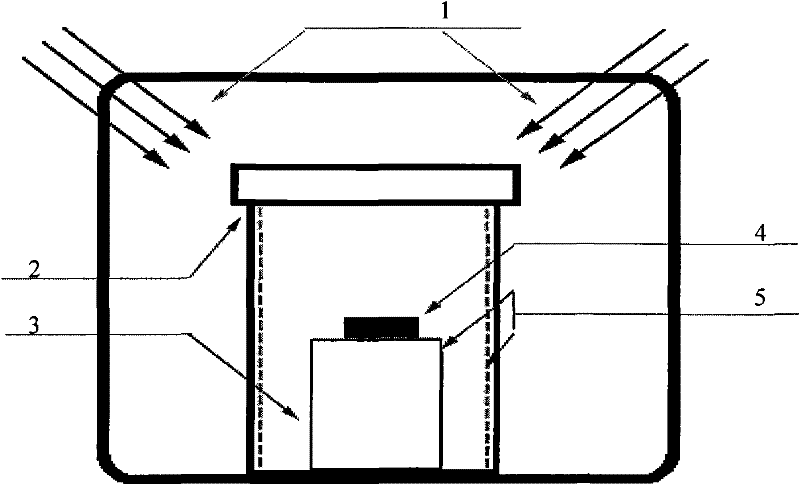
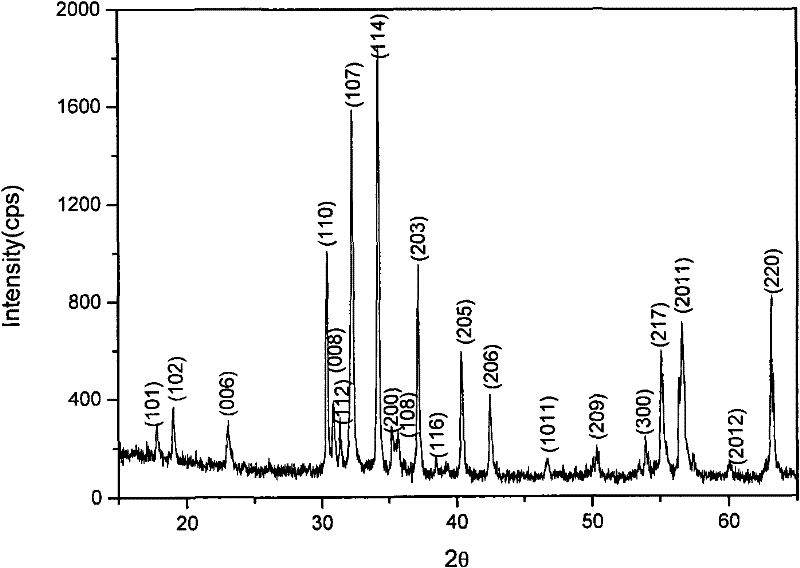
Method of one-step synthesis of hexagonal barium ferrite nanometer crystal by microwave-assistant sol-gel spontaneous combustion
A microwave-assisted, barium ferrite technology, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of difficult control of grain size, agglomeration and adhesion of grains, and avoid difficult control and dispersion of grain size. Good performance, avoid the effect of grain agglomeration and adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Using analytically pure barium nitrate and ferric nitrate as raw materials, press BaFe 12 o 19 The stoichiometric relationship of the middle components means that an appropriate amount of barium nitrate and ferric nitrate are dissolved in an appropriate amount of deionized water to obtain barium nitrate and ferric nitrate solutions, and their concentrations are respectively: [Ba 2+ ]=0.02M, [Fe 3+ ]=0.24M;
[0031] 2) Add complexing agent ethylenediaminetetraacetic acid (EDTA), citric acid (CA) and ethylene glycol (EG) in the above-mentioned solution, and its addition is according to (M EDTA +M CA ) / M Ba+Fe =1.5, M EDTA / M CA = 1 / 6 and M EG / (M EDTA +M CA )=1.2, then stirred until all dissolved. Further, ammonium nitrate was added according to the oxidation degree Q=0.8, and the pH value of the solution was adjusted to 7.0 with ammonia water, and the reaction was refluxed and stirred at 25°C for 2 hours to obtain a completely reacted uniform sol;
[0032] ...
Embodiment 2
[0035] 1) Using analytically pure barium nitrate and ferric nitrate as raw materials, press BaFe 12 o 19 The stoichiometric relationship of the middle components is called an appropriate amount of barium nitrate and ferric nitrate and is dissolved in an appropriate amount of deionized water respectively to obtain barium nitrate and ferric nitrate solutions, and their concentrations are respectively: [Ba 2+ ]=0.01M, [Fe 3+ ]=0.12M;
[0036] 2) Add ethylenediaminetetraacetic acid (EDTA) and urea (Ur) to the solution of barium nitrate and ferric nitrate in the solution, the addition amount is according to M EDTA / M Ba+Fe =1.0, M Ur / M Ba+Fe =1.0 equimolar ratio. Add ammonium nitrate to the solution according to the degree of oxidation Q=1.0, and adjust the pH value of the solution to 6.5 with concentrated ammonia water, then stir and react at room temperature (20° C.) for 2 hours to ensure sufficient reaction, and obtain a completely reacted uniform sol;
[0037] 3) Slowly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com