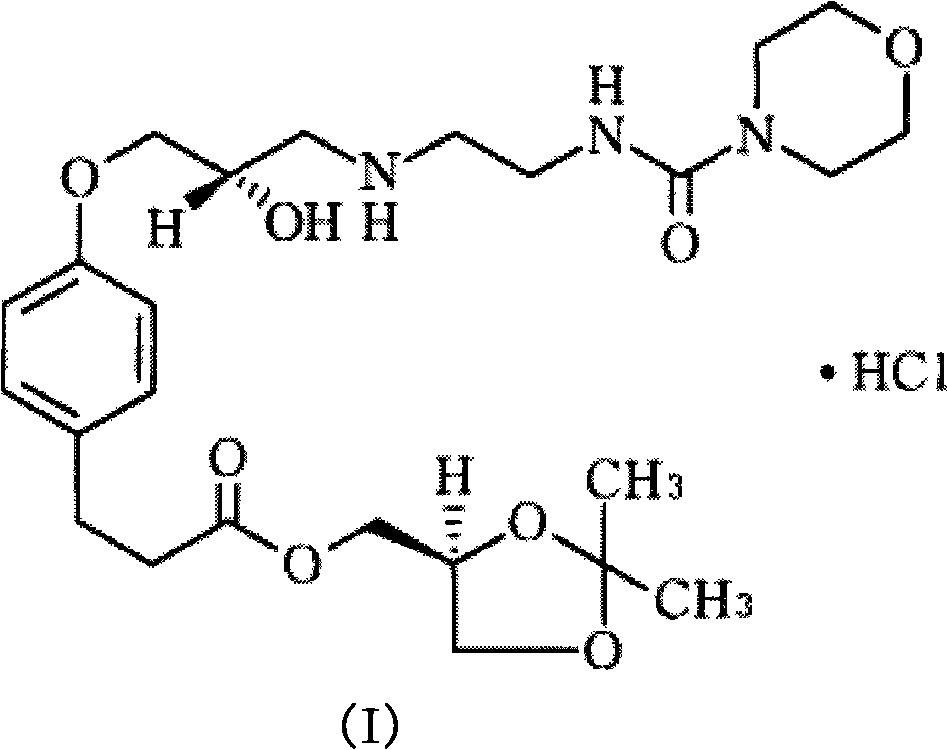
Gamma-crystalline form of landiolol hydrochloride, preparation method of same and pharmaceutical composition containing same
A technology for landiolol hydrochloride and crystal form, which is applied in the field of γ-crystal form of landiolol hydrochloride, its preparation and pharmaceutical composition containing it, can solve the problem of low yield and purity of landiolol hydrochloride. The problem is not high, and the effect of easy industrial scale production is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Get 36g of landyrolol hydrochloride crude product prepared by JP3072475 method, add 360ml acetone, 3.6g gac. Heat to reflux for 15 minutes. Filtrate hot to remove activated carbon. Crystallize below 10°C for 2 hours. Suction filtration, the solid was washed with 80ml acetone. Dry under reduced pressure at 40°C for 6 hours to obtain 29.6 g of γ-crystalline form of landisolol hydrochloride, with a melting point of 124.3°C to 125.7°C and a yield of 82.2%.
[0024] X-ray powder diffraction pattern:
[0025] Determine the X-ray powder diffraction spectrum under the following test conditions:
[0026] Instrument model: Japan Rigaku D / max-rC type anode turning target X-ray instrument
[0027] Conditions: 40kV, 50mA, ray wavelength CuKa DS=SS=1°, RS=0.3mm, scanning range 0-40.0°, scanning rate 5.0° / min.
[0028] The X-ray powder diffraction pattern of Landisolol Hydrochloride γ-crystalline form is given by the important spectral lines organized in the following table: ...
Embodiment 2
[0031] The formula for preparing 1000 lyophilized preparations each containing 50mg landipolol hydrochloride:
[0032] Gamma-crystalline form of Landisolol Hydrochloride 50g
[0033] Mannitol 50g
[0034] Appropriate amount of sodium hydroxide
[0035] Add water for injection to 2000ml
[0036] Prepared according to the conventional process, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com