Nonylphenol polyoxyethylene ether trimeric surfactant using piperazine as connecting group
A technology of nonylphenol polyoxyethylene ether and surfactant, which is applied in the field of non-ionic nonylphenol polyoxyethylene ether trimerized surfactant and its preparation, which can solve equipment corrosion, acid cannot be removed cleanly, increase Product production costs and other issues to achieve the effect of avoiding the formation of by-products, the structure of the synthetic product is accurate, and the route is clear
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Synthesis of Nonylphenol Polyoxyethylene Ether-7 Trimeric Surfactant Tri(NP)-7
[0024] The first step, the synthesis of intermediate 2,6-bis{N-[N'-(2-hydroxyl-5-nonylbenzyl)piperazinyl]methyl}-4-nonylphenol:
[0025] (1) According to the molar ratio, anhydrous piperazine: paraformaldehyde = 1: 2.5 weighs the material, adds in the there-necked flask, then adds n-butanol with 20 times the quality of anhydrous piperazine, and the temperature is at 80°C, and the reaction After 1h, the intermediate N,N'-dimethylolpiperazine was obtained.
[0026] (2) Add commercially available industrial product nonylphenol dropwise to the reactant in the three-necked flask of the previous step reaction to continue the reaction. And keep the temperature of the material at 70°C, react for 3 hours, then introduce the Dean-Stark reactor into the reaction system, react at 120°C for 5 hours to evaporate the water in the system, cool to room temperature, precipitate light yellow crystals, and re...
Embodiment 2
[0031] Synthesis of Nonylphenol Polyoxyethylene Ether-20 Trimeric Surfactant Tri(NP)-20
[0032] The first step, the synthesis of intermediate 2,6-bis{N-[N'-(2-hydroxy-5-nonylbenzyl)piperazinyl]methyl}-4-nonylphenol
[0033] (1) The molar ratio is anhydrous piperazine: paraformaldehyde = 1: 3. Weigh the material, add it to a three-necked flask, then add n-butanol 20 times the mass of anhydrous piperazine, and react for 1 h at a temperature of 80°C. , to obtain the intermediate N, N'-dimethylolpiperazine.
[0034](2) Add commercially available industrial product nonylphenol dropwise to the reactant in the three-necked flask of the previous step reaction to continue the reaction. And keep the temperature of the material at 100°C, react for 3 hours, then introduce the Dean-Stark reactor into the reaction system, react at 110°C for 5 hours, evaporate the water in the system, cool to room temperature, precipitate light yellow crystals, and recrystallize three times from ethanol to...
Embodiment 3~6
[0038] The molar ratio of piperazine to formaldehyde, the molar ratio of nonylphenol to N,N'-dimethylolpiperazine and the number of moles of ethylene oxide added in Examples 3 to 6 are shown in the table below, and other synthetic process steps are implemented in the same way example 1.
[0039]
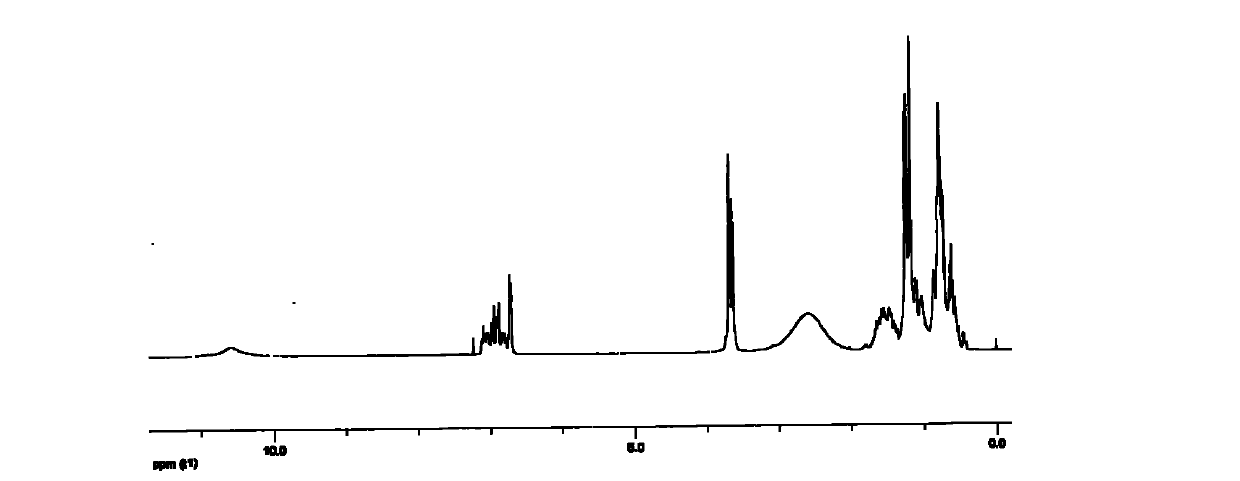
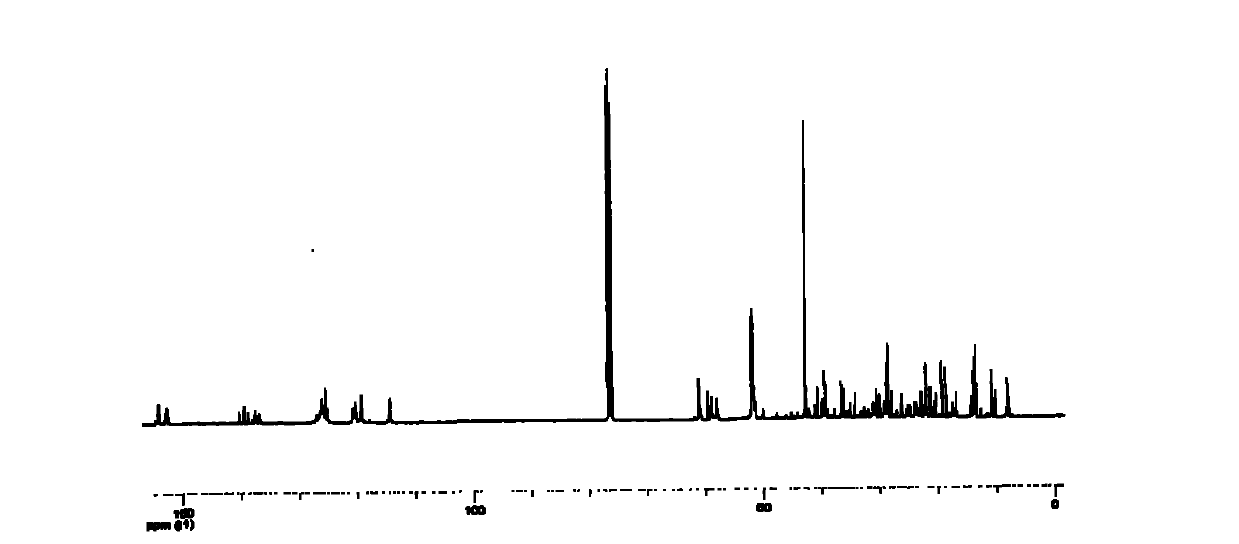
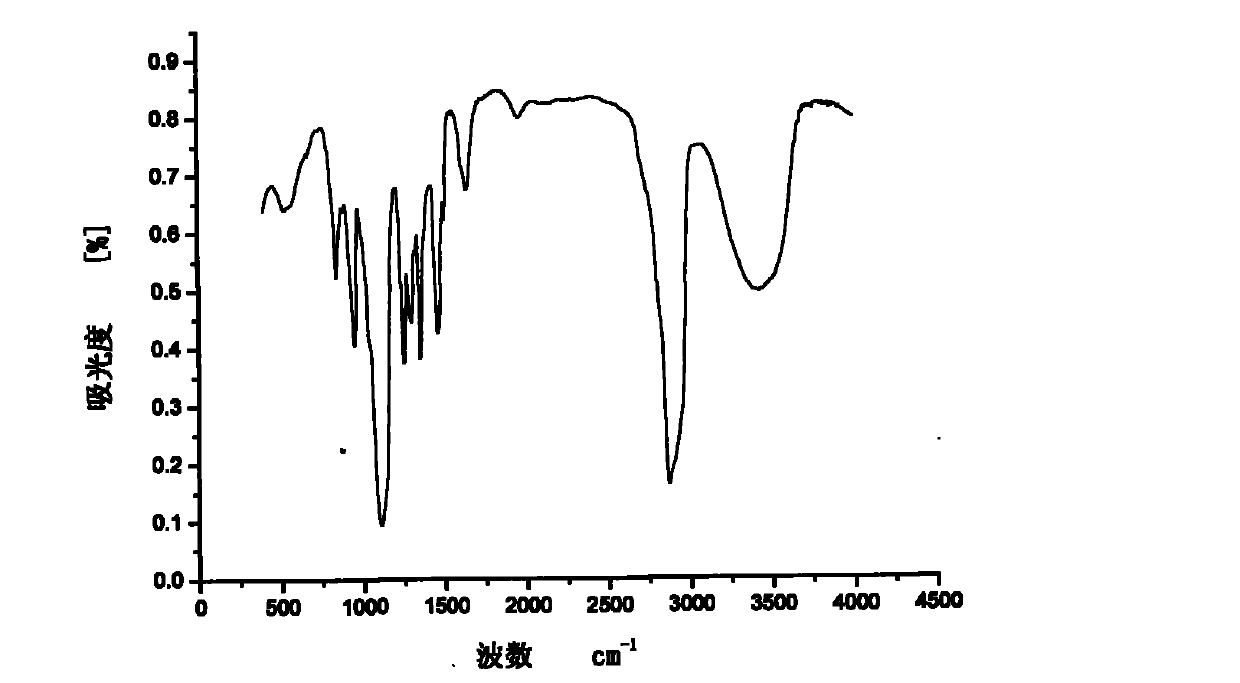
[0040] The above examples illustrate that the present invention adopts the classic Mannich reaction mechanism to synthesize the nonylphenol polyoxyethylene ether dimerized surfactant, and the intermediate is separated and purified by recrystallization, which has the advantages of easy separation and high crystallinity. Since the piperazine as the linking group is a secondary amine, the generation of other by-products can be effectively avoided. The product was characterized by H NMR, C NMR, and IR spectra to accurately analyze the structure of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com