Organic metallic complexes containing phthalocyanine compound and use thereof
A compound and metal technology, applied to phthalocyanine compounds containing organometallic complexes and their application fields, can solve problems such as cost reduction, difficulty in coagulation, coagulation and precipitation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
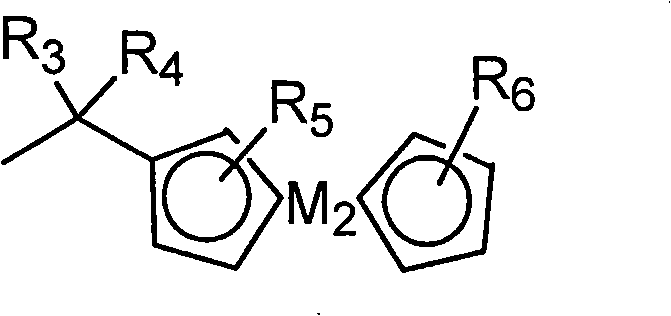
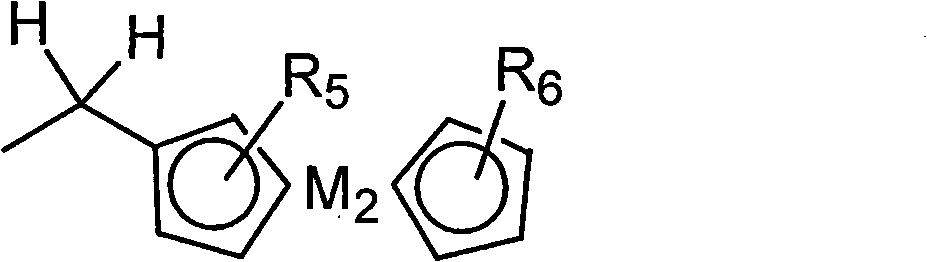
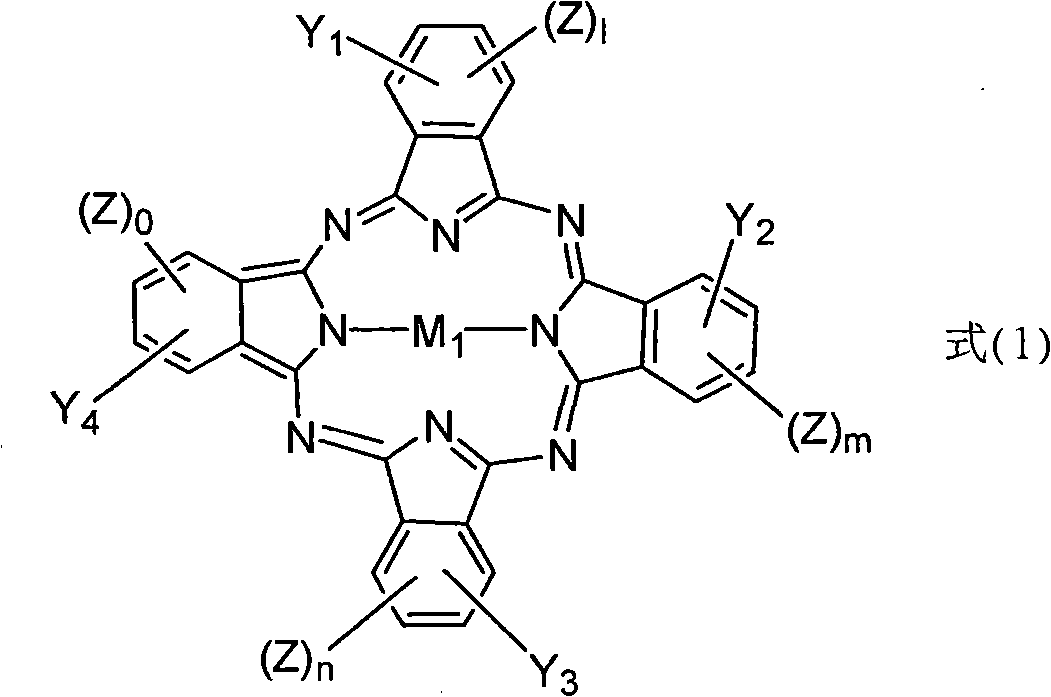
[0109] In a dry reaction flask, tetra(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine compound (tetra-(α-2,4-dimethyl-3-pentyloxy) copper phthalo-cyanine, (referred to as CuPc) 30g was dissolved in 300ml of dichloroethane, stirred and dissolved for 30min. Then take methyl chloride bis (cyclopentadienyl) ferrous compound (Chloromethylferrocene, referred to as FcCH 2 Cl, where Fc represents bis(cyclopentadienyl) ferrous) 12.2g (prepared according to the method of J.Am.Chem.Soc.1966,88,3442 pages) was added, and then, 1.56g of aluminum trichloride was taken Crush and add quickly, stir for 10 minutes after adding, then heat up to 80°C, react for 4 hours, then cool down to room temperature.
[0110] Take another 1L beaker, put 180g of ice cubes and 180ml of water into the mixture, put it into a magnet and stir it. Pour the above reaction solution into it, control the temperature at about 10°C, stir for 1 hour after the addition, pour into the extraction bottle to separate layer...
Embodiment 2
[0118] In a dry reaction bottle, dissolve 30 g of tetra(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine compound in 300 ml of dichloroethane, and stir to dissolve for 30 min. Then take methyl chloride bis(cyclopentadienyl) ferrous compound 12.2g and add, then, take 98% H 2 SO 4 Add 0.86g, stir for 10 minutes after the addition, then heat up to 90°C, react for 3 hours, and then cool down to room temperature.
[0119] Take another 1L beaker, put 60g of ice cubes and 300ml of water into the mixture, put it into a magnet and stir it. Pour the above reaction liquid into it, control the temperature at about 10-15°C, stir for half an hour after the addition, pour into the extraction bottle to separate layers, drain the water layer, extract twice with 300mL of water continuously, and collect the organic layer. Then concentrated under reduced pressure and distilled to about 70g left, then poured into 900ml of methanol solution at 10°C stirred by a mechanical stirrer, a large amount...
Embodiment 3
[0126] In a dry reaction bottle, dissolve 30 g of tetra(α-2,4-dimethyl-3-pentyloxy) copper phthalocyanine compound in 300 ml of dichloroethane, and stir to dissolve for 30 min. Then take 34.1 g of methyl bis(cyclopentadienyl) ferrous chloride and add it, then, take 2.33 g of aluminum trichloride, crush it and add it quickly, stir for 10 minutes after adding, then heat up to 80°C, react 6 hours, and then cooled to room temperature.
[0127] Take another 1L beaker, put 180g of ice cubes and 180ml of water into the mixture, put it into a magnet and stir it. Pour the above reaction solution into it, control the temperature at about 10°C, stir for 1 hour after the addition, pour into the extraction bottle to separate layers, drain the water layer, extract twice with 300mL of water continuously, and collect the organic layer. Then concentrated under reduced pressure and distilled to about 100g left, then poured into 900ml of methanol solution at 10°C stirred by a mechanical stirrer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com