Stable pharmaceutical drug aerosols
A medicament, a technology for preparing a drug, applied in the field of stable drug products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
other Embodiment approach
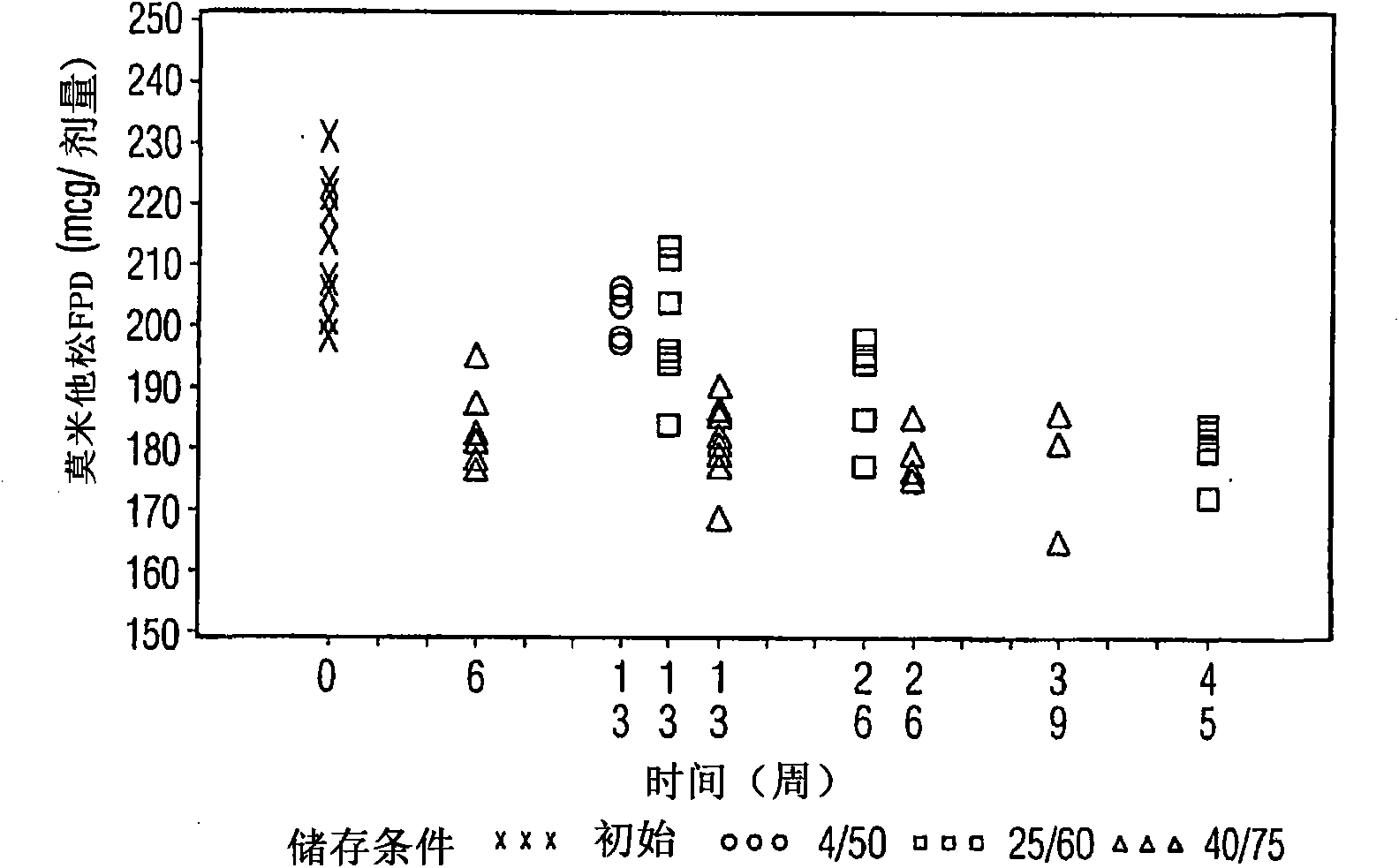
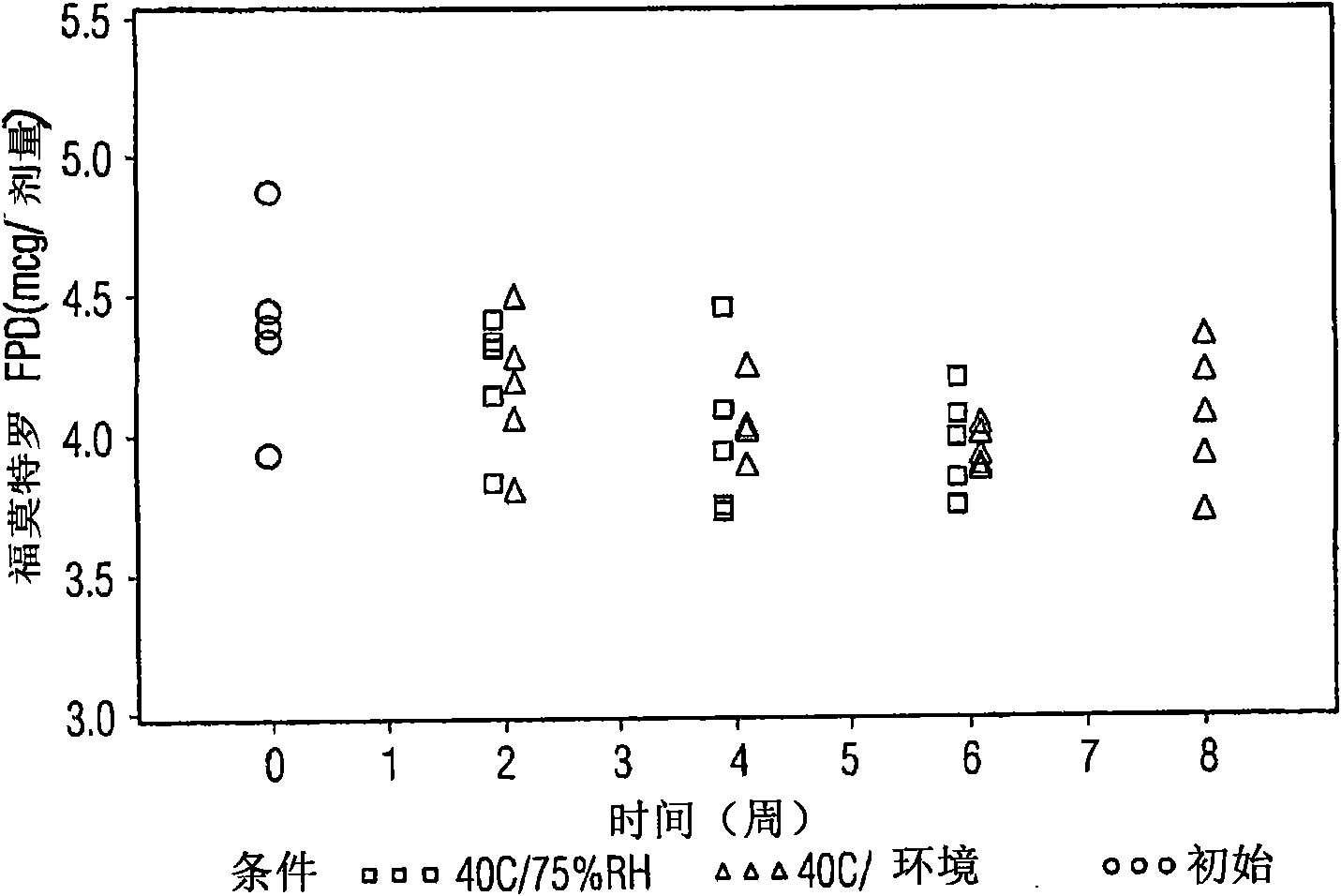
[0010] Other embodiments provide a pharmaceutical product comprising a metered dose inhaler container comprising at least one active agent, ethanol, a propellant. When stored under ambient conditions, the at least one active agent can maintain substantially the same fine particle size for a period of at least about 3 months from the date of manufacture. The at least one active agent may include mometasone furoate and optionally formoterol fumarate. Ambient conditions are considered to include temperatures between about 20°C and 25°C. Desirably, the fine particle fraction varies by no more than about 20%, or by no more than about 15%, or by no more than about 10%, or by no more than about 5%. Useful excipients include co-solvents, surfactants, carriers and combinations of two or more of them. More specifically, useful excipients include lactose, lecithin, oleic acid, and combinations of two or more thereof.
[0011] Various embodiments of the invention provide methods of sta...
Embodiment
[0049] The samples in Tables 1 and 2 were prepared according to the cold fill method. In the cold fill method, chilled propellant HFA 227 is added to a chilled batching vessel with constant agitation. In the concentrate vessel, a mixture of ethanol and oleic acid was prepared and added to the ingredient vessel to form the placebo mixture. Some of the placebo mixture was then transferred from the dosing vessel to a pre-chilled cold concentrate vessel. The active agent is added to the cooled contents in the cold concentrate vessel and mixed. Mix the concentrate in the cold concentrate vessel and transfer it back to the batch vessel. The resulting formulation was continuously mixed and maintained between about -50°C and about -60°C. Dispense the required amount of formulation into suitable canisters, such as aluminum containers coated with FEP on the inside, which are immediately sealed with metering valves. The cells were check weighed and heat stressed. The units were stor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com