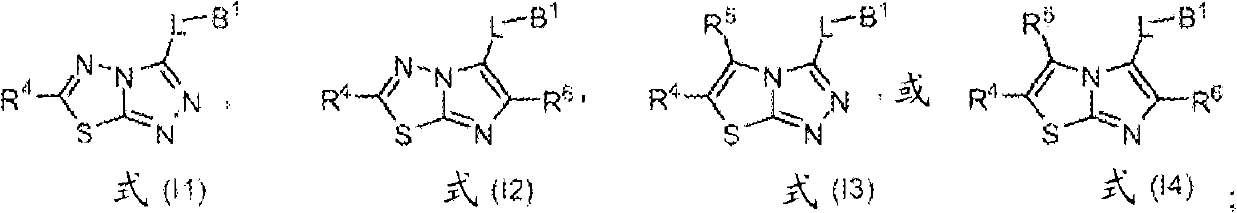
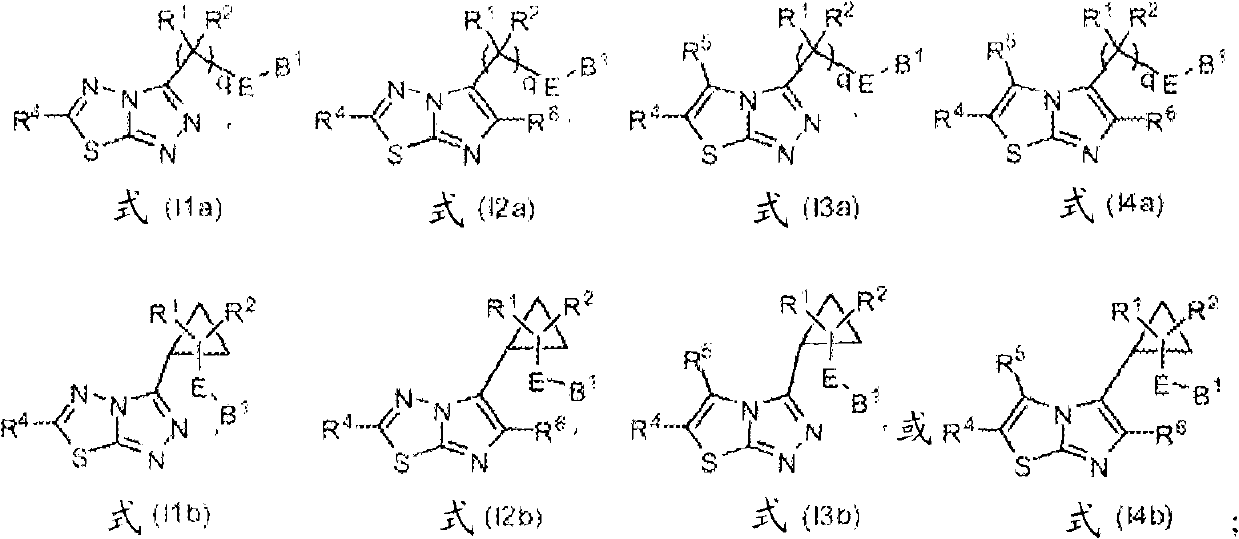
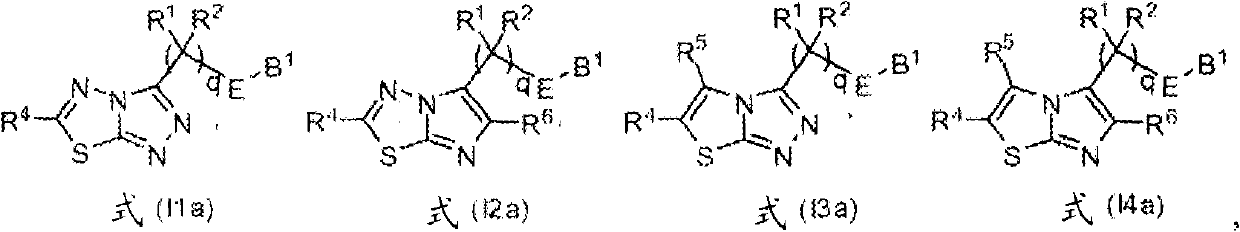
Heterocyclic kinase modulators
A technology of heterocycloalkyl and heteroaryl, applied in the field of inhibitors of homologous proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0817] The following examples illustrate but do not limit the invention. The preparation of embodiments of the invention is described in the following examples. In certain embodiments, the provided chemical reactions and synthetic methods can be modified to prepare various other compounds of the invention. In other embodiments, where compounds of the invention are not exemplified, those of ordinary skill in the art will appreciate that such compounds can be prepared by modification of the synthetic methods provided herein.
[0818] Intermediate 1: (7-Fluoro-quinolin-6-yl)-acetic acid
[0819]
[0820] Step 1: 6-Bromo-7-fluoro-quinoline
[0821] 4-Bromo-3-fluoro-phenylamine (2.85g, 15mmol), ferrous sulfate (0.95g), glycerol (5.658g, 4.5ml), nitrobenzene (1.125g, 0.93ml) and concentrated sulfuric acid ( 2.61 mL) of the mixture was heated slightly. After the first vigorous reaction, the mixture was heated to reflux for 7 hours. The nitrobenzene was evaporated in vacuo. T...
Embodiment 2
[1005] Example 2: General Method A
[1006] Reaction scheme 1
[1007]
[1008] where R 4 Compounds of formula (I) as defined herein are either commercially available or prepared using transformations known to those skilled in the art.
[1009] where L and B 1 Compounds of general formula (II) as defined herein are either commercially available or prepared using the methods described for the synthesis of intermediate 5 and transformations known to those skilled in the art.
[1010] The compound of general formula (III) can be prepared by the compound of formula (I) and the compound of general formula (II) by method step (i), described method comprises aminothiol (II) and formic acid (I) in POCl 3 Heated in presence.
Embodiment 2A
[1011] Example 2A: 6-[6-(1-Methyl-1H-pyrazol-4-yl)-[1,2,4]triazolo[3,4-b][1,3,4]thia Oxadiazol-3-ylmethyl]-quinoline
[1012]
[1013] 4-Amino-5-quinolin-6-ylmethyl-4H-[1,2,4]triazole-3-thiol (0.78g, 3.03mmol), 1-methylpyrazole-4-carboxylic acid (0.39 g, 3.03 mmol) in phosphorus oxychloride (7.5 mL) was refluxed for 6 hours. The reaction mixture was cooled to room temperature, then 30 g of crushed ice were added with stirring, followed by solid potassium hydroxide until the pH of the mixture was 8. The mixture was left to stand in an ice bath for 2 hours. The formed precipitate was filtered, washed with water, placed in boiling ethanol, and refluxed for 30 minutes, then cooled. The resulting solid was filtered and dried to afford the title compound as an off-white solid (195 mg, 18.5%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com