Cytotoxic anti-lag-3 monoclonal antibody and its use in the treatment or prevention of organ transplant rejection and autoimmune disease
A LAG-3, monoclonal antibody technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, antibody medical components, cell culture active agents, etc., can solve problems affecting arterial thrombus stability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] For the production of monoclonal antibodies, any technique that produces antibodies by culturing serial cell lines can be used. Examples include hybridoma technology (9), trioma technology, human B cell hybridoma technology (10).
[0060] The techniques described for the production of single chain antibodies (US Patent No. 4,946,778) can be readily used to produce single chain antibodies to the CD223 polypeptide. Additionally, transgenic mice can be used to express humanized antibodies to immunogenic CD223 polypeptides.
[0061] The first monoclonal antibody of the present invention (referred to as A9H12) is produced by a hybridoma deposited at CNCM on April 27, 2007, with accession number CNCM I-3755.
[0062] The second monoclonal antibody of the present invention (referred to as 31G11) is produced by a hybridoma deposited at CNCM on April 27, 2007 with accession number CNCM I-3756.
[0063] The present invention also relates to the use of the cytotoxic anti-LAG-3 m...
Embodiment 1
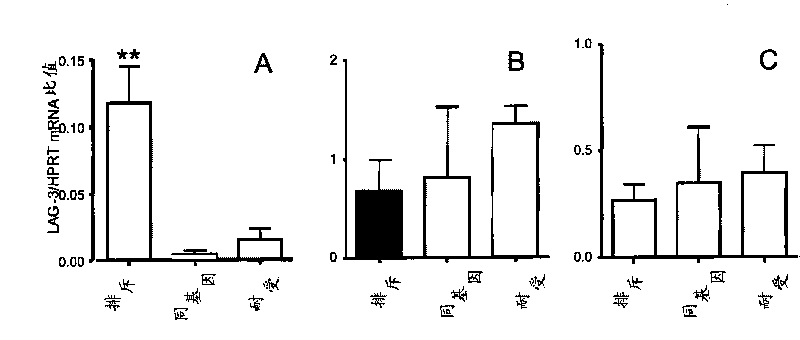
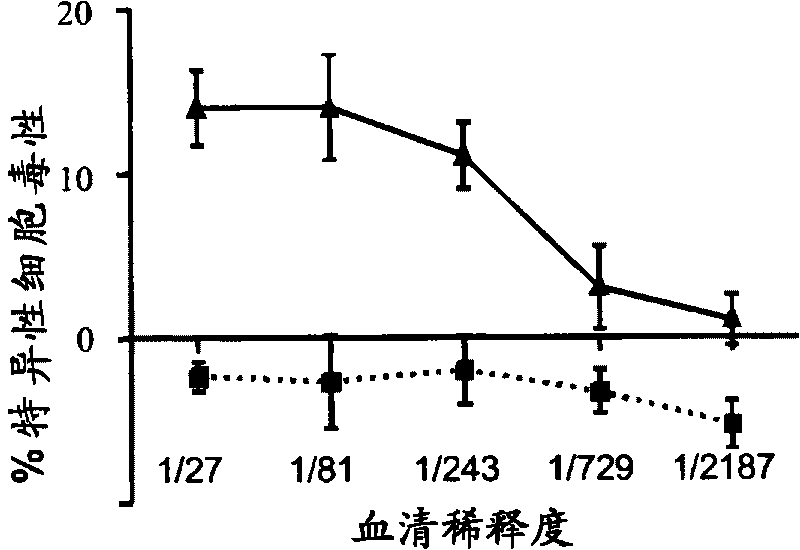
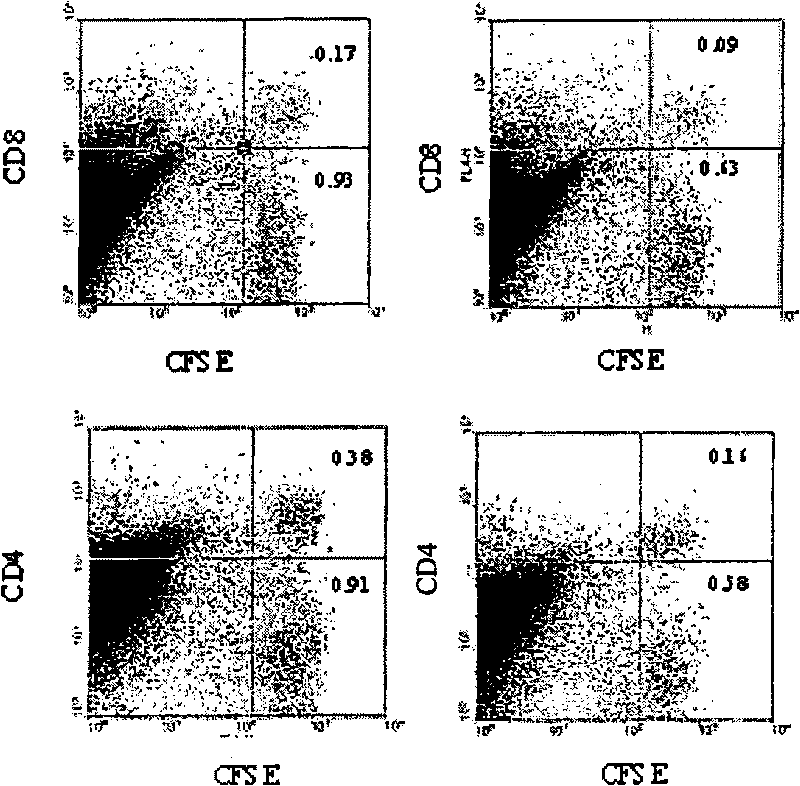
[0069] Example 1: Targeting LAG-3 positive cells with cytotoxic antibodies
[0070] Materials and methods
[0071] Animals and Transplantation
[0072] 8-12 weeks old male Lewis.1W (LEW.1W, haplotype RT1 u ) and Lewis.1A (LEW.1A, haplotype RT1 a ) congenic rats (Centre d'Elevage Janvier, Le Genest-Saint-Isle, France) differ across the entire MHC region. Heterotopic LEW.1W heart transplantation was performed as described above (11). Graft survival was assessed by palpable inspection of the abdominal wall.
[0073] anti-LAG-3 antibody
[0074] A synthetic peptide corresponding to the extra loop domain of the rat LAG-3 protein (NCBI accession number DQ438937; peptide DQPASIPALDLLQGMPSTRRHPPHR) linked to ovalbumin was used to immunize two rabbits. Preimmune and immune sera collected on day 63 (post-4 immunization) were assayed against immunogens and peptides by ELISA and against Con-A activated rat splenocytes by flow cytometry. Preimmune sera were negative in both assa...
Embodiment 2
[0101] Example 2: Generation of novel high affinity hLAG-3 mAbs
[0102] Materials and methods
[0103] CHO cells transfected with hLAG-3 (10 7 cells, intraperitoneal injection) to immunize mice three times, followed by intravenous injection of 10 μg IMP321 (clinical grade hLAG-3Ig recombinant protein) for boost. Three days after the boost, splenocytes were fused with the X63.AG8653 fusion partner to generate hybridoma cells. Hybridoma supernatants were screened for specific binding (FACS analysis) to hLAG-3 transfected CHO versus wild-type CHO cells.
[0104] A mouse IgG2a antibody (580.1E9H3A9H12, referred to as A9H12) was selected, subcloned to generate a stable cell line, and additionally cleared by CDC (complement-dependent cytotoxicity) and ADCC (antibody-dependent cellular cytotoxicity) LAG-3 + The cells were characterized for their potency, considering that the mouse IgG2a Fc region is known to be the most efficient Fc isotype in mice to deliver these activitie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com