Cefixime oral administration mixed suspension and preparation method thereof
A technology for oral suspension and cefixime, which is applied in liquid delivery, pharmaceutical formula, emulsion delivery, etc., to achieve good drug compliance, simple preparation method, and solve stability problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The preparation method of cefixime oral suspension of the present invention, every 100ml of cefixime oral suspension comprises the following steps: one, by the conventional dosage of liquid preparation, methylparaben, ethylparaben, nipagin More than one of propylparaben and butylparaben is dissolved in propylene glycol or / and ethanol; 2. one of povidone K30, povidone K25 and povidone K90 greater than 0 to 20.0g Add more than one kind of liquid maltitol or / and liquid sorbitol, stir to dissolve; three, add more than one of menthol, cherry flavor, strawberry flavor, vanilla flavor, orange flavor and banana flavor, paraben One or more of ester, ethylparaben, propylparaben and butylparaben, mixed with propylene glycol or / and ethanol solution, stirring and mixing evenly; 4. Add 0.5-4.0g cefixime micropowder, liquid maltose Stir and disperse evenly to the required amount of alcohol to obtain cefixime oral suspension; the particle size of the cefixime micropowder is greater tha...
Embodiment 1
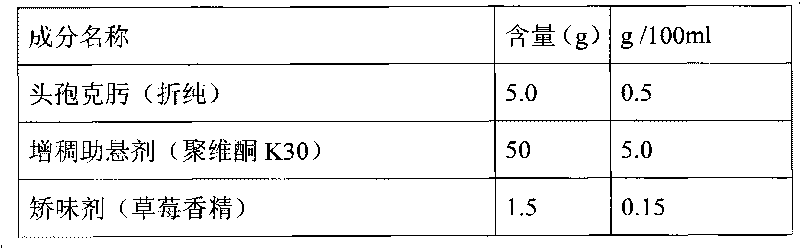
[0028] Embodiment 1, preparation 1000ml cefixime oral suspension (5ml: 25mg)
[0029]
[0030]
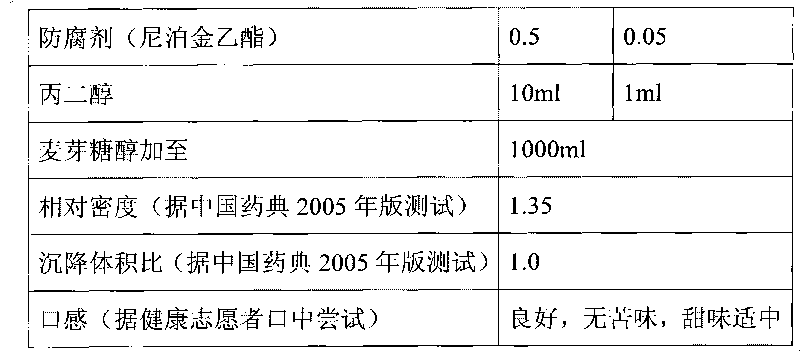
[0031] Preparation process: Dissolve ethylparaben in propylene glycol and set aside; add povidone K30 to an appropriate amount of maltitol and stir to dissolve, then add strawberry essence and propylene glycol solution of ethylparaben, stir and mix evenly, then add Add cefixime micropowder and maltitol to 1000ml, stir at high speed to disperse evenly, and you get it. The particle size of the cefixime micropowder is greater than 0 to 150 μm, the stirring speed is 1000 rpm, and the stirring time is 20 minutes.
Embodiment 2
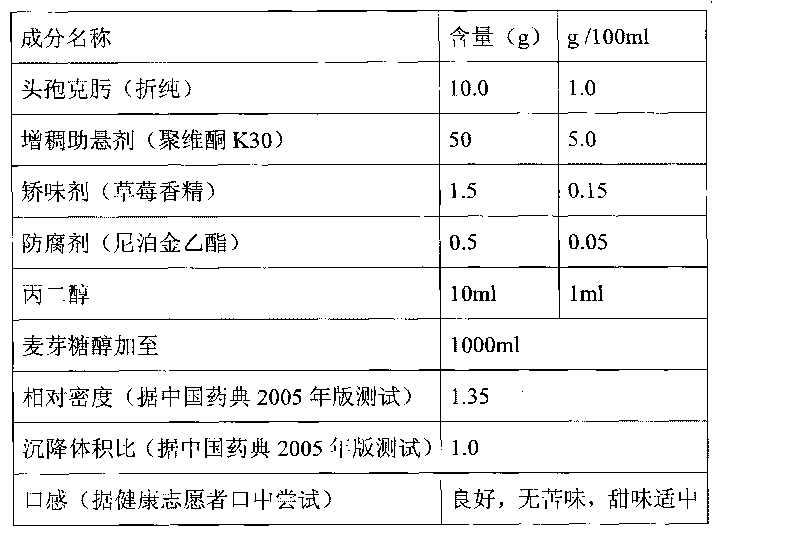
[0032] Embodiment 2, preparation 1000ml cefixime oral suspension (5ml: 50mg)
[0033]
[0034] Preparation process: Dissolve ethylparaben in propylene glycol and set aside; add povidone K30 to an appropriate amount of maltitol and stir to dissolve, then add strawberry essence and propylene glycol solution of ethylparaben, stir and mix evenly, then add Add cefixime micropowder and maltitol to 1000ml, stir at high speed to disperse evenly, and you get it. The particle size of the cefixime micropowder is greater than 0 to 120 μm, the stirring speed is 1500 rpm, and the stirring time is 15 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com