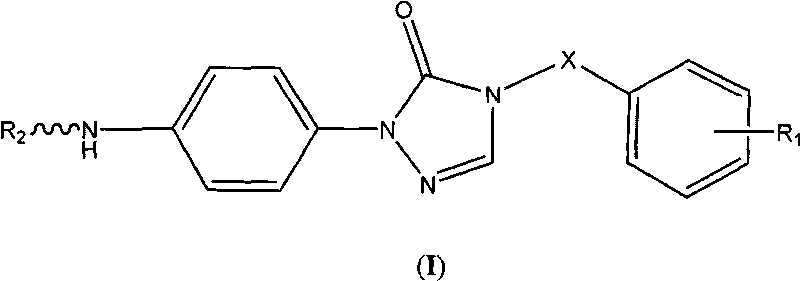
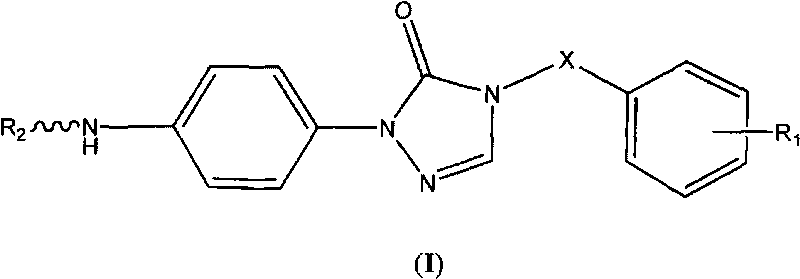
Triazolone compound as well as preparation method and application thereof
A compound and general formula technology, applied in the field of triazolone derivatives and their preparation, can solve the problems of low specificity, poor selectivity, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] 1-(4-aminophenyl)-4,5-dihydro-1,2,4-triazol-5-one (V)
[0054]
[0055] A 100 mL round bottom flask was charged with 2.06 g (10 mmol) of compound IV, 0.1 g of 5% Pd / C catalyst and 60 mL of methanol. The reaction system was hydrogenated at room temperature and pressure for 1 hour with stirring. The catalyst in the reaction mixture was removed by filtration, and the filtrate was evaporated to remove the solvent on a rotary evaporator to obtain colorless crystals, namely product V. 1.72g, 98%. IR(KBr), 3342, 3321, 1645cm -1 .
Embodiment 2
[0057] 1-(4-Aminophenyl)-4-benzyl-4,5-dihydro-1,2,4-triazol-5-one (VII-1)
[0058]
[0059] 1.76g (10mmol) of compound V was added to a 100mL round bottom flask, dissolved in 30mL of dry THF, and 0.4g (10mmol, 60%) of solid NaH was added in batches under stirring, and stirred for half an hour. Then 1.27 g (10 mmol) of BnCl VI-1 was added and stirred overnight at room temperature. The solid in the reaction system was removed by filtration, and the solvent was evaporated from the filtrate on a rotary evaporator to obtain a colorless solid, which was recrystallized from absolute ethanol to obtain product VII-1. Colorless crystals, 2.23g, yield 84%. IR(KBr), 3345, 3328, 1641cm -1 .
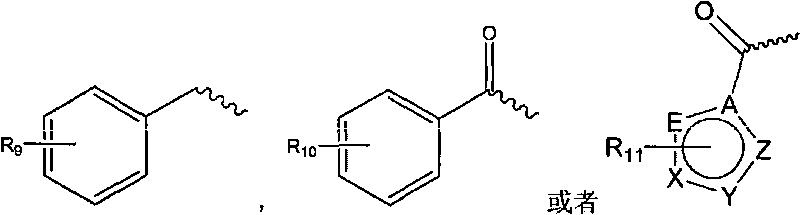
[0060] Compound VI-1 is one of the compounds of general formula VI, likewise compound VII-1 is one of the compounds of general formula VII.
Embodiment 3-17
[0062] Referring to the operation of Example 2, VI-2 to VI-16 in the following table replaced VI-1 in Example 2, and the rest of the operations were the same as in Example 2 to obtain compounds VII-2 to VII-16.
[0063] Compounds VI-2 to VI-16 belong to the compounds of the general formula VI, likewise compounds VII-2 to VII-16 belong to the compounds of the general formula VII.
[0064] Reality
Example sequence
No
Produce
Rate / %
VI
VII
3
82
VI-2: 2-
Chlorobenzyl chloride
VII-2: 1-(4-aminophenyl)-4-(2-chloro)benzyl-4,
5-Dihydro-1,2,4-triazol-5-one
4
83
VI-3: 3-A
yl-2-nitrobenzyl
VII-3: 1-(4-aminophenyl)-4-(3-methyl-2-nitrate
Base) benzyl-4,5-dihydro-1,2,4-triazol-5-one
5
80
VI-4: 3-fluoro
VII-4: 1-(4-aminophenyl)-4-(3-fluoro)benzyl-4, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com