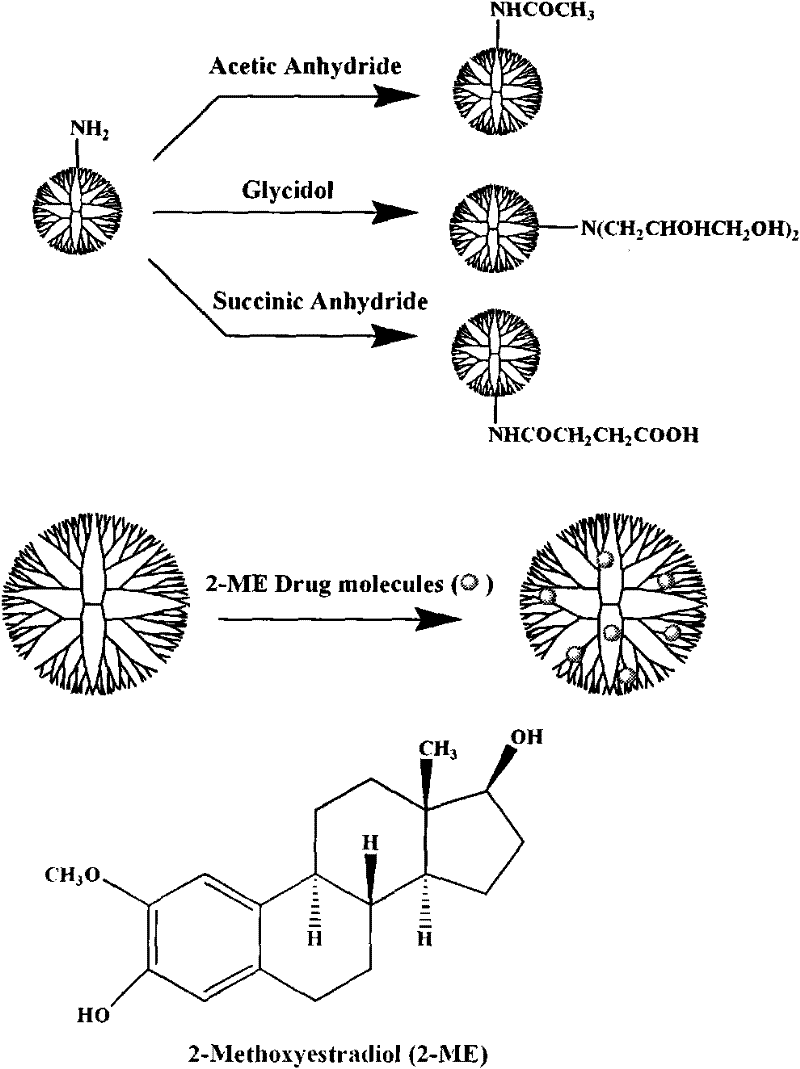
Preparation method of polyamide-amine dendrimer/2-methoxy estradiol compound with terminal functional groups
A kind of technology of polyamidoamine dendrimer and methoxyestradiol, which is applied in the field of preparation of polyamidoamine dendrimer/2-methoxyestradiol compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] (1) 50 milligrams of terminal groups are the fifth generation dendrimers (G5.NH 2 ) was dissolved in 10 ml of dimethyl sulfoxide solution, succinic anhydride (with G5.NH 2 The molar ratio of peripheral amino groups is 2:1 or 3:1) dissolved in 10 ml of dimethyl sulfoxide solution (the dendrimers whose terminal groups are amino groups react with succinic anhydride to generate dendrimers whose terminal groups are carboxyl groups) Macromolecules), mixed two DMSO solutions under vigorous stirring, and reacted at room temperature for 24 hours, then dialyzed with water for 3 days (6 times 4 liters) to remove excess succinic anhydride and organic solvent. Then freeze-dry to obtain carboxylated dendrimer (G5.SAH) solid (yield: 53.6-75.2%);
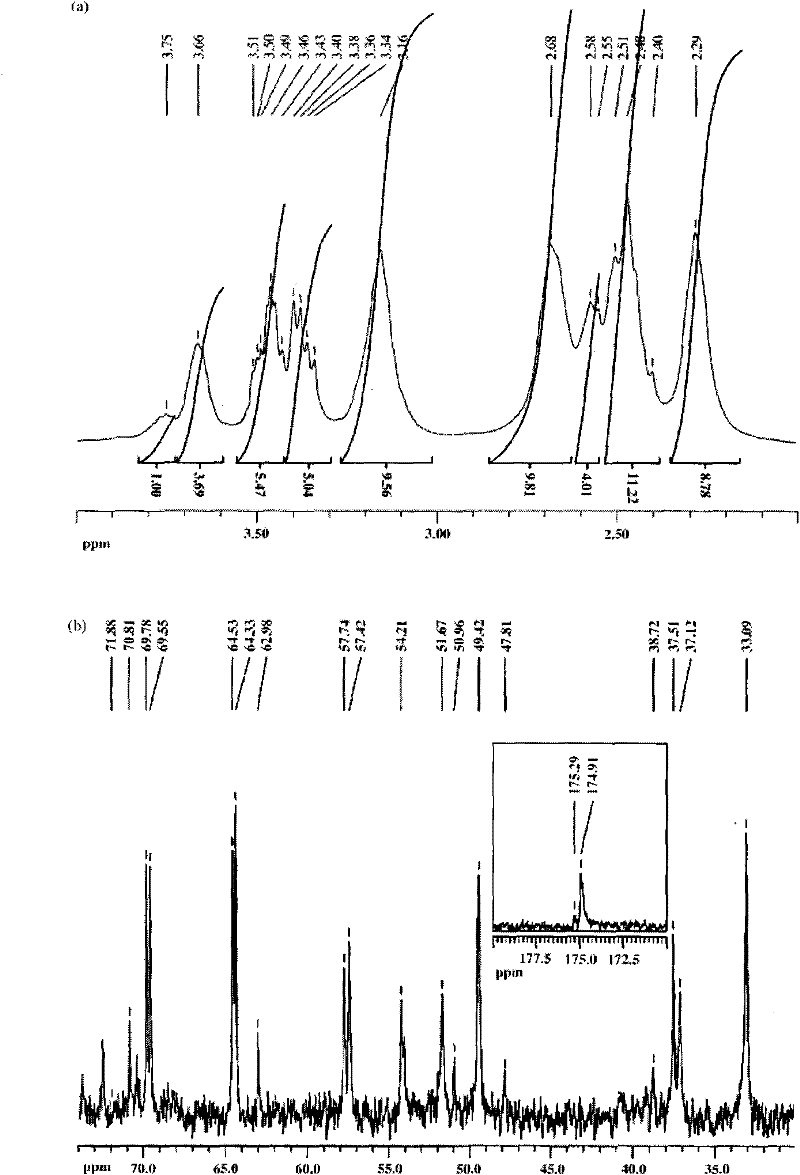
[0044] (2) 10 milligrams of carboxylated dendrimers G5.SAH were dissolved in 1.5 milliliters of water, and 2-ME, which was 10 times the molar equivalent of G5.SAH dendrimers, was dissolved in 300 microliters of methanol, and then the two M...
Embodiment 2
[0046] (1) Add 0.5 ml of triethylamine to a solution of 100 mg of G5.NH 2 10 ml of methanol solution. Will work with G5.NH 2 Acetic anhydride with a molar ratio of 2.5:1 peripheral amino groups was dissolved in 10 ml of methanol solution, and the two methanol solutions were mixed under vigorous stirring. After reacting at room temperature for 24 hours, the methanol was removed with a rotary evaporator, and the resultant was dissolved in water. Dialyzed with water for 3 days (6 times 4 liters) to remove by-products and excess reactants, and then freeze-dried to obtain acetylated dendrimer (G5.Ac) solid (yield: 91.4-94.2%);
[0047] (2) Dissolve 10 mg of acetylated dendrimer G5.Ac in 1.5 milliliters of water, dissolve 2-ME 10 times the molar equivalent of G5.Ac dendrimer in 300 microliters of methanol, and then dissolve the two Mix them together and vigorously stir at room temperature, overnight, evaporate the methanol naturally, centrifuge the mixed solution (7000 rpm, 10 min...
Embodiment 3
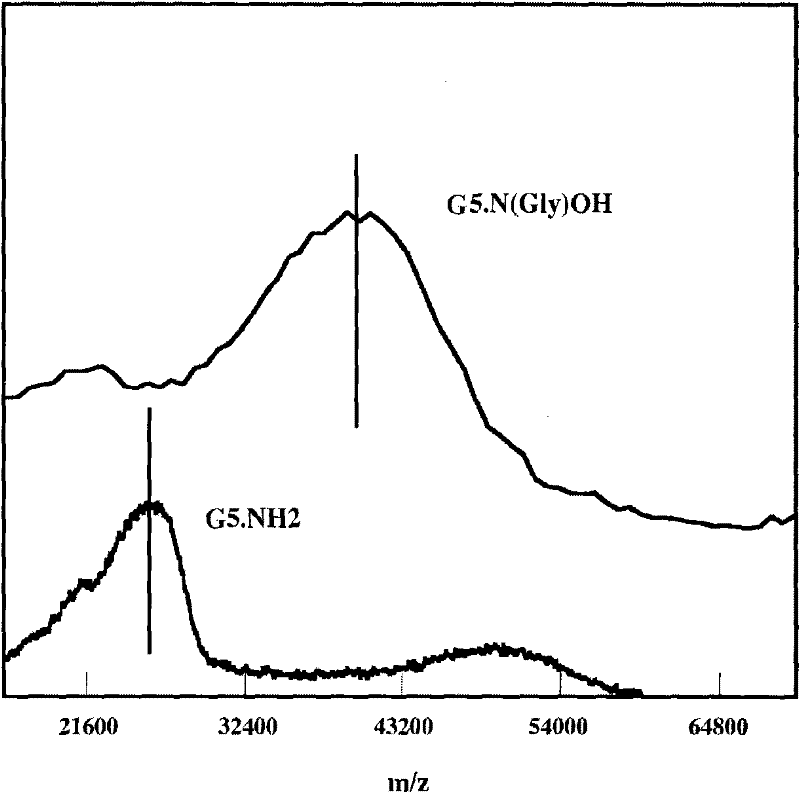
[0049] (1) Add 0.305 grams of G5.NH 2 Dissolve in 20 milliliters of methanol, and add 0.418 grams of glycidol in 10 milliliters of methanol solution (glycidyl alcohol / -NH 2 Molar ratio=4.4:1), after reacting at room temperature for 24 hours, remove methanol with a rotary evaporator, dissolve the resultant in water and dialyze with water for 3 days (6 times 4 liters), remove by-products and excess reactants, and then freeze Dry to obtain 0.45 g of hydroxylated G5.NGlyOH dendrimer solid (yield: 93.9%);
[0050] (2) 10 mg of hydroxylated dendrimer G5.NGlyOH was dissolved in 1.5 milliliters of water, and 2-ME, which was 10 times the molar equivalent of G5.NGlyOH dendrimer, was dissolved in 300 microliters of methanol, and then the two Mix them and stir vigorously at room temperature, overnight, evaporate methanol naturally, centrifuge the mixed solution (7000 rpm, 10 minutes), obtain the supernatant containing G5.NGlyOH / 2-ME complex, and collect the uncoated 2-ME precipitate thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com