Method for preparing drug isradipine for treating hypertension
A technology of isradipine and compounds, applied in the field of compound preparation, can solve the problems of long steps, poor controllability, pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of the present invention is described more specifically below. However, it should be understood that the present invention is not limited to the specific reaction conditions (eg, solvent, amount of compound used, reaction temperature, time required for reaction, etc.) given below.
[0053] In the process of the present invention, each reaction is usually carried out in an inert solvent at room temperature to reflux temperature (such as 0°C to 100°C, preferably 0°C to 80°C). The reaction time is usually 0.1-60 hours, preferably 0.5-48 hours.
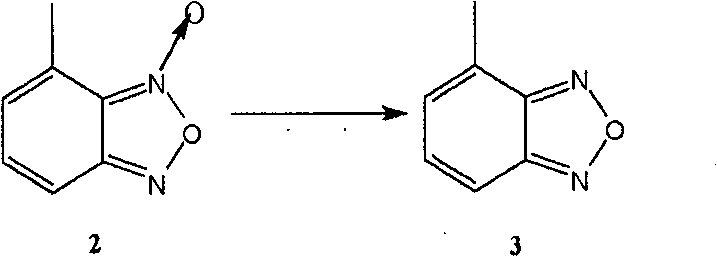
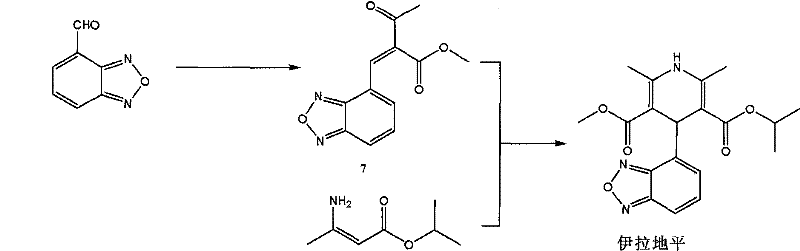
[0054] The preparation method of the present invention can be represented by the following flow process:
[0055]
[0056] The following is a more specific description of process 1:
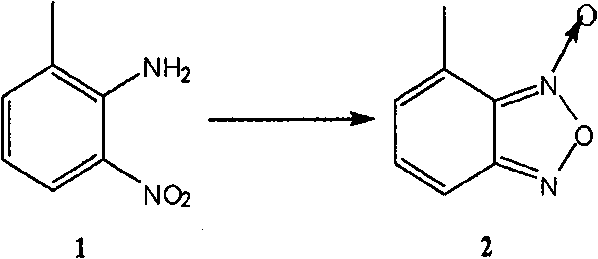
[0057] (a). 2-Amino-3-methylnitrobenzene is oxidized and ring-closed to form 4-methylbenzofurazan oxide (compound 2)
[0058] In this step, usable inert solvents include methanol, ethanol, isopropanol, water, or any combin...
Embodiment 14
[0106] Embodiment 14-methylbenzofurazan oxide (compound 2)
[0107] Add compound 1 (22.8g, 0.15mol) to a 1L three-necked round-bottomed flask, then add KOH (10g) in ethanol solution (200ml), add NaClO solution (100ml) dropwise under ice cooling, and continue the reaction under ice-water bath . After the reaction is complete, CH is added to the system 2 Cl 2 (150ml) was stirred, and the insoluble matter was filtered off, and the filtrate was separated into an organic phase, anhydrous Na 2 SO 4 Dry and concentrate. 20.77 g of crude product were obtained. Ethyl acetate was added for recrystallization to obtain 15.5 g of yellow needle-like crystals, with a yield of 68.9%.
Embodiment 24
[0108] Embodiment 24-methylbenzofurazan oxide (compound 2)
[0109] (a) Compound 1 (30.4g, 0.2mol), water (80mL) and concentrated hydrochloric acid (45mL) were added to a 500mL three-neck round bottom flask, and after cooling in an ice bath, an aqueous solution of sodium nitrite (14.5g, 0.21mol) was added dropwise (50 mL), after the dropwise addition, keep stirring for 1 hour.
[0110] (b) Suction filtration, pour the filtrate into a 1L beaker, add sodium azide (13g, 0.2mol) in batches under ice bath conditions, after adding, keep stirring for 1 hour, suction filtration, wash the filter cake with water (100mL, 2 times ).
[0111] (c) drop the filter cake obtained in the (b) process into a three-necked round-bottomed flask equipped with toluene (60mL), slowly heat up to reflux, and reflux for 5 hours, the system is cooled to room temperature, and the insolubles are removed by suction filtration, and the filtrate is concentrated. The residue was cooled and crystallized, and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com