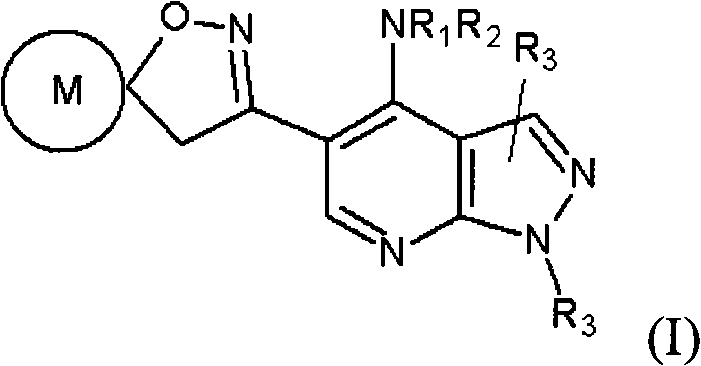
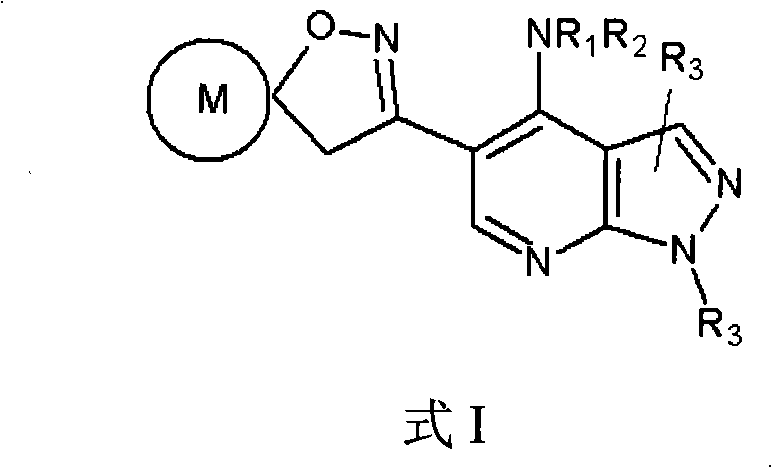
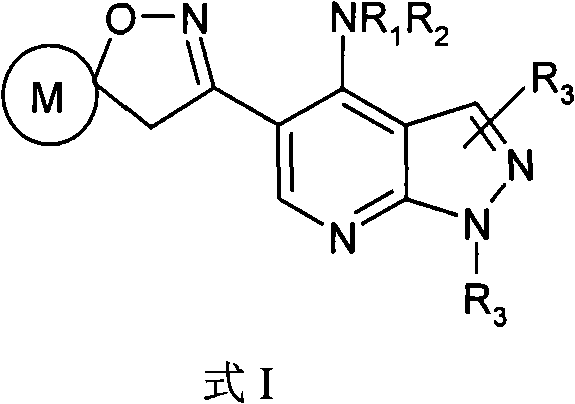
Pyrazolo (3, 4-b) pyridine derivatives as phosphodiesterase inhibitors
一种吡唑并、4-b的技术,应用在制备所公开的化合物领域,能够解决难以克服、剂量依赖性副作用、限制等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[0618] Example 1a: Preparation 1- ( 4-methoxybenzyl)-1H-pyrazol-5-amine
[0619] The compound is according to Bioorganic and medicinal chemistry letters (bioorganic and medicinal chemistry), 13, 1133-1136 (2003) described method is synthesized.
Embodiment 1b
[0620] Example 1b: Preparation of 1-ethyl-3-methyl-1H-pyrazol-5-amine
[0621] The compound according to Chem.Pharm.Bull. 52 (9), 1098-1104 (2004) described in the method for synthesis.
Embodiment 1c
[0622] Example 1c: Preparation of tetrahydro-2H-pyran-4-amine hydrochloride
[0623] The compound according to Tetrahedron letters, 42 , 4257-4259, (2001) described in the method for synthesis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com